Evaluation of bioreactors with and without air injection for the micropropagation of Vanilla planifolia G. Jackson
DOI:
https://doi.org/10.29312/remexca.v16i6.3804Keywords:
liquid media, mechanical, micropropagation, Rita®, vanillaAbstract
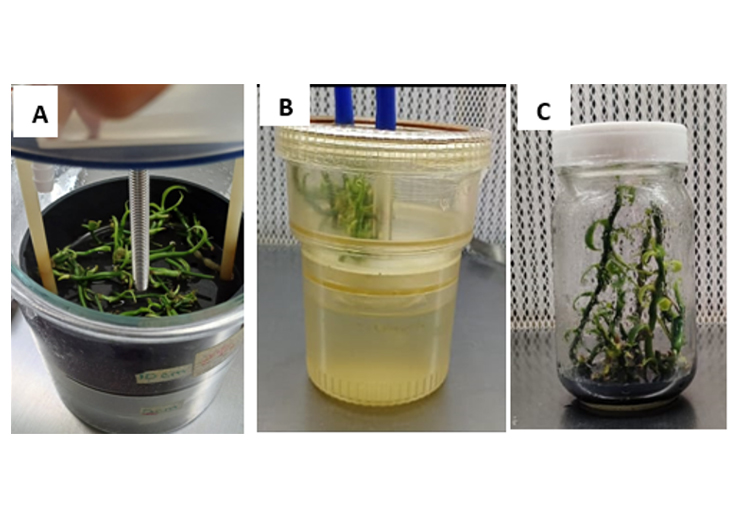
The species Vanilla planifolia G. Jackson has a high commercial value in the food, pharmaceutical and cosmetic industries. Temporary immersion systems or bioreactors allow for faster and more controlled in vitro propagation under laboratory conditions. Nonetheless, due to the high costs of commercial bioreactors, such as the Rita® model, one of the most widely used for the micropropagation of several plant species, cheaper alternatives are sought. In vitro multiplication of vanilla was carried out in two types of temporary immersion bioreactors in order to evaluate the efficiency of a mechanical bioreactor without air injection compared to a Rita® bioreactor that uses air injection; the research was conducted between 2023 and 2024. The semi-solid culture system was used as a control. After 30 days of culture, the following physiological variables were analyzed: number of shoots, number of leaves, shoot length, and growth index; likewise, biochemical variables, such as total contents of chlorophylls α and β and phenol and carbohydrate contents, were quantified. The results obtained indicated that the BWA bioreactor was statistically equal (p≤ 0.05) to the Rita® bioreactor in the variables of number and length of shoots. Both were statistically different (p≤ 0.05) from the semi-solid system in most of the variables assessed. This suggests that the use of a mechanical bioreactor without air injection can be used as an alternative for the micropropagation of various species due to its low cost.
Downloads
References
Aragón, C. E.; Escalona, M.; Capote, I.; Pina, D.; Cejas, I.; Rodríguez, R.; Jesús-Cañal, M.; Sandoval, J.; Roels, S.; Debergh, P. and González-Olmedo, J. 2005. Photosynthesis and carbon metabolism in plantain (Musa AAB) plantlets growing in temporary immersion bioreactors and during ex vitro acclimatization. In Vitro Cellular & Developmental Biology-Plant. 41(4):550-554. https://doi.org/10.1079/IVP2005640.
Arencibia, A. D.; Vergara, C.; Quiroz, K.; Carrasco, B.; Bravo, C. and Garcia-Gonzales, R. 2013. an approach for micropropagation of blueberry (Vaccinium corymbosum L.) plants mediated by temporary immersion bioreactors (TIBs). American Journal of Plant Sciences. 4(5):1022-1028. https://doi.org/10.4236/ajps.2013.45126.
Debabrata, S.; Ramesh, C. and Prakash, S. N. 1997. Effect of inoculation density on potato micropropagation. Plant Cell, Tissue and Organ Culture. 48(1):63-66.
Dewir, D. H.; Chakrabarty, D.; Hahn, D. and Paek, E. J. 2006. A simple method for masspropagation of Spathiphylium cannifolium using an airlift bioreactor. In vitro Cellular & Developmental Biology-Plant. 42(3):291-297.
Escalona, M.; Lorenzo, J. C.; González, B.; Daquinta, M.; Gonzalez, J. L.; Desjardins, Y. and Borroto, C. G. 1999. Pineapple (Ananas comosus L. Merr.) micropropagation in temporary immersion systems. Plant Cell Reports. 18(9):743-748. https://doi.org/10.1007/s002990050653.
Escalona, M.; Samson, G.; Borroto, C. and Desjardins, Y. 2003. Physiology of effects of temporary immersion bioreactors on micro propagated pineapple plantlets. In vitro Cellular & Developmental Biology Plant. 39(6):651-656. https://doi.org/10.1079/IVP2003473.
Etienne, H. and Berthouly, M. 2002. Temporary immersion systems in plant micropropagation. Plant Cell, Tissue and Organ Culture. 69:215-231.
Etienne, H.; Lartaud, M.; Michaux-Ferriere, N.; Carron, M. E.; Berthouly, M. and Teisson, A. 1997. Improvement of somatic embryogenesis in Hevea brasiliensis (Mt) ll. Arg.) using the temporary immersion technique. In vitro Cellular & Developmental Biology- Plant. 33(2):81-87.
Gao, J. and Lee, J. M. 1992. Effect of oxygen supply on the suspension culture of genetically modified tobacco cells. Biotechnology Progress. 8(4):285-290. https://doi.org/10.1021/bp00016a004.
Georgiev, V.; Schumann, A.; Pavlov, A. and Bley, T. 2014. Temporary immersion systems in plant biotechnology. Engineering in Life Sciences. 14(6):607-621. https://doi.org/10.1002/elsc.201300166.
Hahn, E. J. and Paek, K. Y. 2005. Multiplication of Chrysanthemum shoots in bioreactors as affected by culture method and inoculation density of single node stems. Plant Cell, Tissue and Organ Culture. 81(3):301-306. https://doi.org/10.1007/s11240-004-6655-0.
Jin, M. Y.; Piao, X. C.; Xiu, J. R.; Park, S. Y. and Lian, M. L. 2013. Micropropagation using a bioreactor system and subsequent acclimatization of grape rootstock ‘5BB’. Scientia Horticulturae. 164:35-40. https://doi.org/10.1016/j.scienta.2013.09.004.
Jova, M. C.; Kosky, R. G. and Cuellar, E. E. 2011. Effect of liquid media culture systems on yam plant growth (Dioscorea alata L. Pacala Duclos). Biotechnology, Agronomy, Society and Environment. 15(4):515-521.
Lichtenthaler, H. K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Lester Packer and Roland Douce. Methods in Enzymology, Plant Cell Membranes. 148:350-382. Academic Press. https://doi.org/10.1016/0076-6879(87)48036-1.
Lotfi, M. and Werbrouck, S. P. O. 2020. SETISTM, a novel variant within the temporary immersion bioreactors. Acta Horticulturae. 30(1285):253-258. https://doi.org/10.17660/ActaHortic.2020.1285.37.
Murashige, T. and Skoog, F. 1962. Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 15(3):473-497. https://doi:10.1111/j.1399-3054.1962.tb08052.x.
Robert, M. L.; Herrera-Herrera, J. L.; Herrera-Herrera, G.; Herrera-Alamillo, M. Á. and Fuentes-Carrillo, P. 2006. A new temporary immersion bioreactor system for micropropagation. In: Loyola-Vargas, V. M. and Vázquez-Flota, F. Methods in Molecular Biology. 318(2):121-130. Humana Press. https://doi.org/10.1385/1-59259-959-1:121.
Roels, S.; Escalona, M.; Cejas, I.; Noceda, C.; Rodriguez, R.; Canal, M. J.; Sandoval, J. and Debergh, P. 2005. Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell, Tissue and Organ Culture. 82(1):57-66. https://doi.org/10.1007/s11240-004-6746-y.
Singleton, V. L. and Rossi, J. A. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 82(1):57-66. https://doi.org/10.1007/s11240-004-6746-y.
Trauger, M.; Hile, A.; Sreenivas, K.; Shouse, E. M.; Bhatt, J.; Lai, T.; Mohandass, R.; Tripathi, L.; Ogden, A. J. and Curtis, W. R. 2022. CO2 supplementation eliminates sugar-rich media requirement for plant propagation using a simple inexpensive temporary immersion photobioreactor. Plant Cell, Tissue and Organ Culture (PCTOC). 150(1):57-71. https://doi.org/10.1007/s11240-021-02210-3.
Whitman, F. H.; Blaydes, D. F. and Devlin, R. M. 1971. Experiments in plant physiology. Monograph Wageningen University, Ed. Van Nostrand Rteinhold. New York. 245 p.
Wu, H. C.; Kuo, M. L. and Chen, C. M. 2018. Promotion of vegetative growth in force-ventilated Protea Cynaroides L. explants cultured in modified temporary immersion culture vessels. HortScience. 53(2):231-235. https://doi.org/10.21273/hortsci12513-17.
Wu, S. Q.; Lian, M. L.; Gao, R.; Park, S. Y. and Piao, X. C. 2011. Bioreactor application on adventitious root culture of Astragalus membranaceus. In vitro Cellular & Developmental Biology- Plant. 47(6):719-724. https://doi.org/10.1007/s11627-011-9376-1.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


