Degradation of reserve starch from the seed of wild and domesticated Phaseolus vulgaris L.
DOI:
https://doi.org/10.29312/remexca.v16i3.3602Keywords:
beans, germination, reducing sugars, reserve substanceAbstract
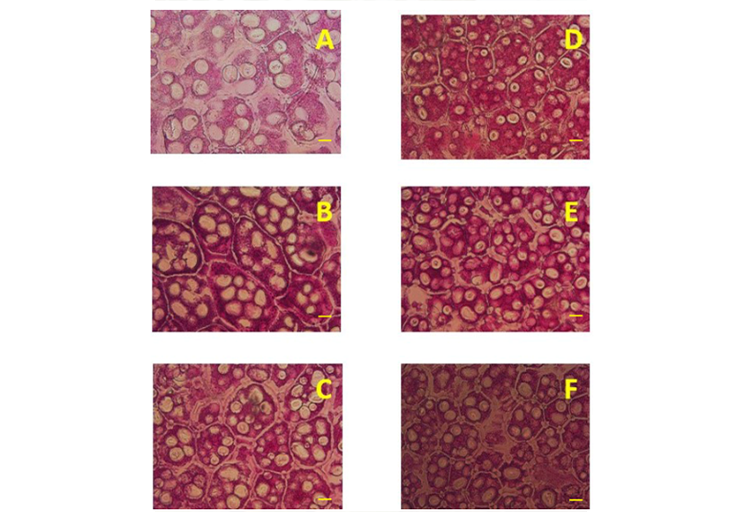
Domestication has modified the size and type of seed reserves; this raises the question of whether these modifications have had an impact on metabolism and the mobilization of these reserves during germination and seedling establishment. The research aimed to determine the effect of domestication on cotyledons, embryonal axis and dark-grown seedlings of domesticated and wild beans. In 2019, eight seeds of three improved varieties and three wild collections were germinated at 25 °C, another eight seeds with exposed radicles were sown in grow bags and the seedlings obtained were kept for 14 days in darkness. The germinated seeds and seedlings were dissected into their structures and their dry mass and the concentrations of starch, glucose, fructose and sucrose were determined; likewise, the number of cells and the number of starch granules mm-2 and their dimensions were also determined in cotyledons. The design used was completely randomized with four replications. On average, the improved varieties had 11 and three times more dry matter in cotyledons and embryonal axis compared to wild varieties, and seven, nine and 13 times more dry matter in root, shoot and remnant of cotyledons, respectively. Concentrations of starch, glucose, and sucrose per gram of dry mass were higher in cotyledons from improved varieties; in contrast, concentrations of starch, glucose, and fructose were higher in the embryonal axis of the wild ones. In the root, wild varieties had higher concentrations of starch, fructose, and sucrose, and in the shoot, domesticated ones had more glucose, fructose, and sucrose. Wild varieties had 42% more cells and 30% more starch granules than domesticated ones per unit area. Domestication modifies the composition and mobilization of reserves during germination and seedling establishment.
Downloads
References
Allaby, R. G. 2020. Domestication syndrome in plants. In: Encyclopedia of Global Archeology. Smith, C. Ed. Springer. New York. 2182-2184 pp. Doi.org/10.1007/978-3-030-30018-0-2416.
Allende-Arrarás, G.; Acero-Godínez, M. G.; Padilla-Ramírez, J. S.; Mayek-Pérez, N. 2006. Comportamiento agronómico y características fisicoquímicas del grano de frijol en Aguascalientes, México. Revista Fitotecnia Mexicana. 29(1):89-93.
Ansari, O.; Chogazardi, H. R.; Sharifzadeh, F. and Nazarli, H. 2012. Seed reserve utilization and seedling growth of treated seeds of mountain rye (Secale montanum) as affected by drought stress. Cercetari Agronomice in Moldova. 45(2):43-48. https://repository.iuls.ro/xmlui/handle/20.500.12811/2297.
Bajaj, R.; Singh, N.; Kaur, A. and Inouchi, N. 2018. Structural, morphological, functional and digestibility properties of starches from cereals, tubers and legumes: comparative study. Journal of food Science and Technology. 55(9):3799-3808. https://doi.org/10.1007/s13197-018-3342-4.
Bewley, J. D.; Bradford, K. J. H.; Henk, W. M.; Nonogaki, H. 2013. Seeds: physiology of development and germination. Springer Science+Business Media LLC. New York. 444 p.
Carbonero, P.; Iglesias-Fernández, R. and Carbajosa, J. V. 2017. The AFL subfamily of B3 transcription factors: evolution and function in angiosperm seeds. Journal of Experimental Botany. 68(4):871-880. Doi:10.1093/jxb/erw458.
Celis-Velázquez, R.; Peña-Valdivia, C. B.; Trejo-López, C.; Aguirre-Rivera, J.R.: Córdova-Téllez, L. and Carballo-Carballo, A. 2008. Consumo de reservas de la semilla de frijol para la emergencia y desarrollo inicial en diferentes profundidades de siembra. Agronomía Mesoamericana. 19(2):167-177. https://www.redalyc.org/pdf/437/43711425002.pdf.
Cilia-García, M.; Peña-Valdivia, C. B.; Bernal Gracida, L. A.; Yáñez Jiménez, P.; García Esteva A. and Padilla-Chacón, D. 2021. Effects of water restriction on carbohydrates concentration, starch granules size and amylolytic activity in seeds of Phaseolus vulgaris L. and P. acutifolius A. Gray. Botanical Sciences. 99(2):364-376. Doi.org/10.17129/botsci.26476.
Coelho, C. M. M. and Benedito, V. A. 2008. Seed development and reserve compound accumulation in common bean (Phaseolus vulgaris L.). Seed Science Biotechnology. 2(2):42-52.
Di Vittori, V.; Gioia, T.; Rodríguez, M.; Bellucci, E.; Bitochi, E.; Nanni, M.; Attene, G.; Rou, D. and Papa, R. 2019. Convergent evolution of the seed shattering trait. Genes. 10(1):68. Doi:10.3390/genes10010068. 16 p.
Estrada-Gómez, J. A.; Estrada-Trejo, V.; Hernández-Livera, A.; Molina-Moreno, J. C. and Campos-Escudero, A. 2004. OTI una nueva variedad de frijol para el Valle de México. Revista Fitotecnia Mexicana. 27(1):115-116.
Golan, G.; Oksenberg, A. and Peleg, Z. 2015. Genetic evidence for differential selection of grain and embryo weight during wheat evolution under domestication. Journal of Experimental Botany. 66(19):5703-5711. https://doi.org/10.1093/jxb/erv249.
Hu, X. W.; Zhang, R.; Wu, Y. P. and Baskin, C. C. 2017. Seedling tolerance to cotyledon removal varies with seed size: a case of five legume species. Ecology and Evolution. 7(15):5948-5955. Doi.org/10.1002/ece3.3169.
Johansen, D. A. 1940. Plant microtechnique. McGraw Hill Book Company, Inc. London. 530 p.
Lastdrager, J.; Hanson, J. and Smeekens, S. 2014. Sugars signals and the control of plant growth and development. Journal of Experimental Botany. 65(3):799-807. Doi:10.1093/jxb/ert474.
Lépiz-Ildefonso, R.; López-Alcocer, J. J.; Sánchez-González, J. J.; Santacruz-Ruvalcaba, F.; Nuño-Romero, R. y Rodríguez-Guzmán, E. 2010. Características morfológicas de formas cultivadas, silvestres o intermedias de frijol común de hábito trepador. Revista Fitotecnia Mexicana. 33(1):21-28.
Milla, R. and Matesanz, S. 2017. Growing larger with domestication: a matter of physiology, morphology or allocation? Plant Biology. 19(3):475-483. https://doi.org/10.1111/plb.12545.
Mohammadi, H.; Soltani, A.; Sadeghipour, H. R. and Zeinaly, E. 2011. Effects of seed aging on subsequent seed reserve utilization and seedling growth in soybean. International Journal of Plant Production. 5(1):65-70.
Morales-Santos, M. E.; Peña-Valdivia, C. B.; García-Esteva, A.; Aguilar-Benítez, G. and Kohashi-Shibata, J. 2017. Características físicas y de germinación en semillas y plántulas de frijol (Phaseolus vulgaris L.) silvestre, domesticado y su progenie. Agrociencia. 51(1):43-62. https://www.scielo.org.mx/pdf/agro/v51n1/1405-3195-agro-51-01-00043-en.pdf.
Ortega-Delgado, M. L. y Rodríguez-Coquíez, C. 1979. Estudio de carbohidratos en variedades mexicanas de frijol (Phaseolus vulgaris L. y Phaseolus coccineus L.). Agrociencia. 37:33-49.
Pandey, R.; Vijay, P. and Dadlani, M. 2010. Mobilization of seed reserves and environmental control of seed germination. In: Seed Science and Technology. Singhal, N.C. Ed. Kalyani Publishers. New Deli, India. 84-116 pp.
Pritchard, S. L.; Charlton, W. L.; Baker, A. and Graham, I. A. 2002. Germination and storage reserve mobilization are regulated independently in Arabidopsis. The Plant Journal. 31(5):39-647. https://onlinelibrary.wiley.com/doi/pdf/10.1046/j.1365-313X.2002.01376.x.
Punia, S.; Dhull, S. B.; Sandhu, K. S.; Kaur, M. and Purewal, S. S. 2020. Kidney bean (Phaseolus vulgaris) starch: A review. Legume Science. 2(3):e52. https://doi.org/10.1002/ leg3.52.
Roucou, A.; Violle, C.; Fort, F.; Roumet, P.; Ecarnot, M. and Vile, D. 2018. Shifts in plant functional strategies over the course of wheat domestication. Journal Applied Ecology. 55:25-37. Doi.org/10.1111/1365-2664.13029.
Sánchez-Linares, L.; Gavilanes-Ruíz, M.; Díaz-Pontones, D.; Guzmán-Chávez, F.; Calzada-Alejo, V.; Zurita-Villegas, V.; Luna-Loaiza, V.; Moreno-Sánchez, R.; Bernal-Lugo, I. and Sánchez-Nieto, S. 2012. Early carbon mobilization and radicle protrusion in maize germination. Journal of Experimental Botany. 63(12):4513-4526. https://doi.org/10.1093/jxb/ers130.
SAS Institute Inc. 2012. SAS version 9.3. Cary, N.C., USA.
Schneider, A.; Aghamirzaie, D.; Elmarakeby, H.; Poudel, A. N.; Koo, A. J.; Heath, L. S.; Grene, R. and Collakova, E. 2016. Potential targets of viviparous 1/abi 3‐like 1 (val 1) repression in developing Arabidopsis thaliana embryos. The Plant Journal. 85(2):305-319.
Shi, J. and Lai, J. 2015. Patterns of genomics change with crop domestication and breeding. Current Opinion in Plant Biology. 24:47-53. Doi.org/10.1016/j.pbi.2015.01.008.
Shi, J. and Lai, J. 2015. Patterns of genomics change with crop domestication and breeding. Current Opinion in Plant Biology. 24:47-53. https://doi.org/10.1016/j.pbi.2015.01.008.
SigmaPlot Version 14. 2019. Systat Software, Inc., San Jose, California.
Singh, S. P.; Gepts, P. and Debouck, D. G. 1991. Races of common bean (Phaseolus vulgaris, Fabaceae). Economic Botany. 45(3):379-396.
Smýkal, P.; Nelson, M. N.; Berger, J. D. and Von Wettberg, E. J. B. 2018. The impact of genetic changes during crop domestication. Agronomy. 8(7):119-141. Doi.org/10.3390/agronomy8070119.
Vargas-Vázquez, M. L. P.; Uscanga-Mortera, E.; Padilla-Chacón, D.; Vibrans, H.; Kohashi-Shibata, J.; Miranda-Colín, S. y Yáñez-Jiménez, P. 2020. Asignación de biomasa y carbohidratos en semillas y plántulas de Phaseolus coccineus L. domesticado y silvestre. Botanical Sciences. 98(2):366-376. Doi: 10.17129/botsci.2485.
Viola, R. and Davies, H. V. 1992. A microplate reader assay for rapid enzymatic quantification of sugars in potato tubers. Potato Research. 35:55-58. https://link.springer.com/content/pdf/10.1007/BF02357723.pdf.
Wani, I. A.; Sogi, D. S.; Wani, A. A.; Gill, B. S. and Shivhare, U. S. 2010. Physico‐chemical properties of starches from Indian kidney bean (Phaseolus vulgaris) cultivars. International Journal of Food Science & Technology. 45(10):2176-2185. http://dx.doi.org/10.1111/j.1365-2621.2010.02379.x.
Yamaguchi, J. 1978. Respiration and growth efficiency in relation to crop productivity. Journal of the Faculty of Agriculture, Hokkaido Univ. 59(1):59-129. https://eprints.lib.hokudai.ac.jp/dspace/handle/2115/12920.
Yoshida, H.; Nozaki, K.; Hanashiro, I.; Yagi, F.; Ito, H.; Honma, M.; Matsui, H. and Takeda, Y. 2003. Structure and physicochemical properties of starches from kidney bean seeds at immature, premature and mature stages of development. Carbohydrate Research. 338(5):463-469. Doi.org/10.1016/S0008-6215(02)00489-5.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


