Inhibitory activity of alcoholic extracts of ediblefungi against Rhizoctonia solani
DOI:
https://doi.org/10.29312/remexca.v14i4.3200Keywords:
Lactarius, Ustilago, phytopathogenAbstract
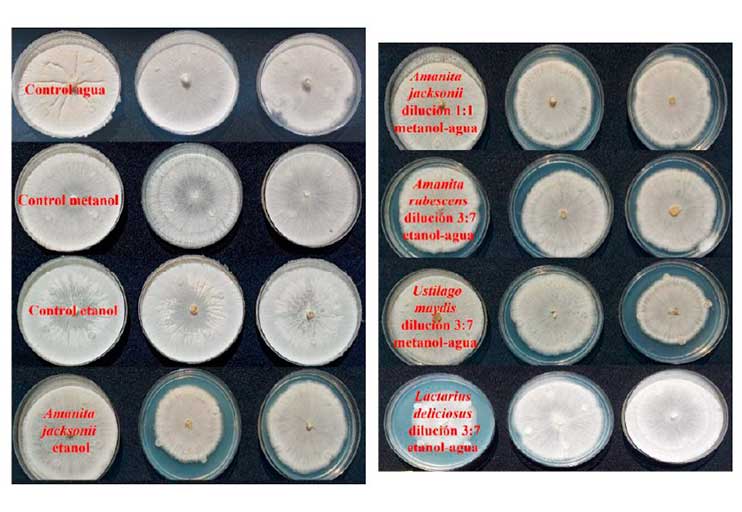
Fungal diseases represent one of the causes of annual crop losses. Rhizoctonia solani is a pathogenic fungus with worldwide distribution associated with root and tuber diseases of different crops; it causes important economic losses in perennial and annual plants, including almost all horticultural crops. To mitigate losses due to fungus, fungicides of synthetic origin have been used; however, many of these substances are associated with carcinogenicity and are toxic to the environment. Edible macromycete mushrooms are a source of antifungal compounds to control diseases in agricultural crops. This work evaluated the antifungal activity of extracts of edible mushrooms (Lactarius deliciosus, Ustilago maydis, Amanita jacksonii and Amanita rubescens) against the phytopathogen R. solani by means of three techniques: diffusion of wells, discs and plate dilution. The results show that with the diffusion of wells, no effect was observed on the growth of R. solani with the alcoholic extracts and their dilutions. While with disc diffusion, a qualitatively slower growth was observed compared to the controls of R. solani with the ethanolic extract of A. jacksonii and in its 1:1 dilution of methanol-water. In the plate dilution, it was found that the 1:1 ethanol-water dilution of L. deliciosus had 88% inhibition on the growth of R. solani, followed by the ethanol extract of L. deliciosus (65%), methanol extract from U. maydis (63%) and methanol extract from L. deliciosus (57%). Therefore, the extracts and the dilution must be studied in greater depth, by being tested in an in vivo system and identifying the secondary metabolites present.
Downloads
References
Adhikari, P.; Pandey, A.; Agnihotri, V. and Pande, V. 2018. Selection of solvent and extraction method for determination of antimicrobial potential of Taxus wallichiana Zucc. Res Pharmacy. 8(1):1-9. Doi: 10.25081/rip.2018.v8.3487.
Ajayi-Oyetunde, O. O.; and Bradley, C. A. 2018. Rhizoctonia solani: taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 67(1):3-17. https://doi.org/10.1111/ppa.12733. DOI: https://doi.org/10.1111/ppa.12733
Akpi, U. K.; Odoh, C. K.; Ideh, E. E. and Adobu, U. S. 2017. Antimicrobial activity of Lycoperdon perlatum whole fruit body on common pathogenic bacteria and fungi. Afr. J. Clinical Exp. Microbiol. 18(2):79-85. Doi: 10.4314/ajcem.v18i2.4. DOI: https://doi.org/10.4314/ajcem.v18i2.4
Al-Askar, A. A.; and Rashad, Y. M. 2010. Efficacy of some plant extracts against Rhizoctonia solani on pea. J. Plant Protec. Res. 50(3):239-243. Doi: 10.2478/v10045-010-0042-0. DOI: https://doi.org/10.2478/v10045-010-0042-0
Alves, M. J.; Ferreira, I. C.; Froufe, H. J.; Abreu, R. M. V.; Martins, A. and Pintado, M. 2013. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 115(2):346-357. https://doi.org/10.1111/ jam.12196. DOI: https://doi.org/10.1111/jam.12196
Arcos, J. y Zúñiga, D. 2015. Efecto de rizobacterias en el control de Rhizoctonia solani en el cultivo de papa. Ecología Aplicada. 14(2):95-101. DOI: https://doi.org/10.21704/rea.v14i1-2.86
Balouiri, M.; Sadiki, M. and Ibnsouda, S. K. 2016. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharmaceutical Analysis. 6(2):71-79. DOI: https://doi.org/10.1016/j.jpha.2015.11.005
Casaril, K. B. P. B.; Kasuya, M. C. M. and Vanetti, M. C. D. 2011. Antimicrobial activity and mineral composition of shiitake mushrooms cultivated on agricultural waste. Braz. Archiv. Biol. Technol. 54(5):991-1002. DOI: https://doi.org/10.1590/S1516-89132011000500017
Cortes, S. A.; Hernández S. H. and Jaramillo, F. M. 2011. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr. J. Microbiol. Res. 5(17):2512-2523. Doi: 10.5897/AJMR10.814. DOI: https://doi.org/10.5897/AJMR10.814
Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clinical Infec. Dis. 26(1):1-10. DOI: https://doi.org/10.1086/516284
Cruz, C. A.; Rodríguez, N. N. and Rodríguez, C. E. 2010. Evaluación in vitro del efecto antibacteriano de los extractos de Bidens pilosa, Lantana camara, Schinus molle y Silybum marianum. Rev. UDCA Actualidad & Divulgación Científica. 13(2):117-124.
Doğan, H. H. and Akbaş, G. 2013. Biological activity and fatty acid composition of Caesar’s mushroom. Pharmaceutical Biol. 51(7):863-871. https://doi.org/10.3109/13880209. 2013.768272. DOI: https://doi.org/10.3109/13880209.2013.768272
Elbatrawy, E. N.; Ghonimy, E. A.; Alassar, M. M. and Wu, F. S. 2015. Medicinal mushroom extracts possess differential antioxidant activity and cytotoxicity to cancer cells. Inter. J. Med. Mushrooms. 17(5):471-479. DOI: https://doi.org/10.1615/IntJMedMushrooms.v17.i5.70
Feussi, T. M.; Qin, J.; Ndongo, J. T. and Laatsch, H. 2017. New azulene-type sesquiterpenoids from the fruiting bodies of Lactarius deliciosus. Natural products and bioprospecting. 7(3):269-273. https://doi.org/10.1007/s13659-017-0130-1. DOI: https://doi.org/10.1007/s13659-017-0130-1
FRAC. 2017. Fungicide Resistance Action Committee. Fungicides sorted by mode of action including FRAC code numbering. http://www.frac.info/docs/default-source/publications/ fraccode-list/frac-code-list-2017-final.pdf?sfvrsn=2.
Frank. J. A. and Leach S. S. 1980. Comparison of tuber-borne and soilborne inoculum in the Rhizoctonia disease of potato. Phytopathology. 70(1):51-53. DOI: https://doi.org/10.1094/Phyto-70-51
Hide, G. A.; Hirst, J. M. and Stedman, O. J. 1973. Effects of black scurf (Rhizoctonia solani) on potatoes. Annal. Appl. Biol. 74(2):139-148. https://doi.org/10.1111/j.1744-7348.1973. tb07733.x. DOI: https://doi.org/10.1111/j.1744-7348.1973.tb07733.x
Khaledi, N.; Taheri, P. and Tarighi, S. 2015. Antifungal activity of various essential oils against Rhizoctonia solani and Macrophomina phaseolina as major bean pathogens. J. Appl. Microbiol. 118(3):704-717. https://doi.org/10.1111/jam.12730. DOI: https://doi.org/10.1111/jam.12730
Li, C.; and Oberlies, N. H. 2005. The most widely recognized mushroom: chemistry of the genus Amanita. Life Sci. 78(5):532-538. https://doi.org/10.1016/j.lfs.2005.09.003. DOI: https://doi.org/10.1016/j.lfs.2005.09.003
Martínez, E. N. A.; Vázquez, G. F. J.; Valero, G. J.; Álvarez, P. E.; Garza, O. F.; Najera, M. J. A. and Quiñónez, M. M. 2021. Antimicrobial activity, phenolic compounds content, and antioxidant capacity of four edible macromycete fungi from Chihuahua, México. TIP. Rev. Especial. Cienc. Quím. Biológ. 24:1-11 https://doi.org/10.22201/fesz.23958723e. 2021.318. DOI: https://doi.org/10.22201/fesz.23958723e
Naczk, M.; and Shahidi, F. 2006. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharmaceutical Bio. Analys. 41(5):1523-1542. https://doi.org/10.1016/ j.jpba.2006.04.002. DOI: https://doi.org/10.1016/j.jpba.2006.04.002
Patiño, M.; Nieto, R. I. J.; Chegwin, A. C. and Torres, R. E. 2020. Actividad biocontroladora in vitro de macrohongos contra diferentes hongos fitopatógenos. Acta Biol. Colomb. 25(2):265-279. https://doi.org/10.15446/abc.v25n2.75303
Priya, K.; Thiribhuvanamala, G.; Kamalakannan, A. and Krishnamoorthy, A. S. 2019. Antimicrobial activity of biomolecules from mushroom fungi against Colletotrichum capsici (Syd) Butler and bisby, the fruit rot pathogen of Chilli. Inter. J. Current Microbiol. Appl. Sci. 8(6):1172-1186.
Rafiq, M.; Javaid, A. y Shoaib, A. 2021. Antifungal activity of methanolic leaf extract of Carthamus oxycantha against Rhizoctonia solani. Pak. J. Bot. 53(3):1133-1139. http://dx.doi.org/10.30848/PJB2021-3(17).
Reinoso, R.; Cajas, M. D.; Martinez, M.; Martin, A.; Perez, C.; Fajardo, V. and Becerra, J. 2013. Biological activity of macromycetes isolated from Chilean subantarctic ecosystems. J. Chilean Chem. Soc. 58(4):2016-2019. DOI: https://doi.org/10.4067/S0717-97072013000400024
Ren, L.; Hemar, Y.; Perera, C. O.; Lewis, G.; Krissansen, G. W. and Buchanan, P. K. 2014. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioactive Carbohydrates and Dietary Fibre. 3(2):41-51. https://doi.org/10. 1016/j.bcdf.2014.01.003. DOI: https://doi.org/10.1016/j.bcdf.2014.01.003
SAS. 2020. Institute Inc. The System for Windows, version 9.4; SAS Institute Inc.: Cary, NC, USA.
Shen, H. S.; Shao, S.; Chen, J. C. and Zhou, T. 2017. Antimicrobials from mushrooms for assuring food safety. Comprehensive reviews in Food Science and Food Safety. 16(2):316-329. https://doi.org/10.1111/1541-4337.12255. DOI: https://doi.org/10.1111/1541-4337.12255
Sommer, N. F.; Fortalage, J. R; Edwuards, D. C. 2002. Postharvest Diseases of selected commodities. In: Kader, A. A. Ed. Postharvest technology of horticultural crops. University of California. 223-227 pp.
Tsror, L. 2010. Biology, epidemiology and management of Rhizoctonia solani on potato. J. Phytopathol. 158(10):649-658. https://doi.org/10.1111/j.1439-0434.2010.01671.x. DOI: https://doi.org/10.1111/j.1439-0434.2010.01671.x
Volcão, L. M.; Halicki, P. C. B.; Christ, R. A.; Ramos, D. F.; Badiale, F. E.; Andreazza, R. and Silva J. F. 2021. Mushroom extract of Lactarius deliciosus (L.) Sf. Gray as biopesticide: antifungal activity and toxicological analysis. J. Toxicol. Environ Health. 85(2):43-55.
Yim, H. S.; Chye, F. Y.; Koo, S. M.; Matanjun, P.; How, S. E. and Ho, C. W. 2012. Optimization of extraction time and temperature for antioxidant activity of edible wild mushroom, Pleurotus porrigens. Food and Bioproducts Processing. 90(2): 235-242. DOI: https://doi.org/10.1016/j.fbp.2011.04.001
Zavastin, D. E.; Miron, A.; Gherman, S. P.; Boerescu, C. M.; Breaban, I. G. and Gavrilescu, C. M. 2015. Antioxidant activity, total phenolic and metals contents of Lactarius salmonicolor. Farmacia. 63(5):755-759.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


