Phytochemical screening and antibacterial effect of phenolic extracts from two Mediterranean Cupressus
DOI:
https://doi.org/10.29312/remexca.v13i5.2473Keywords:
Cupressus arizonica, Cupressus sempervirens, Pseudomonas aeruginosa, hydroalcoholic extractAbstract
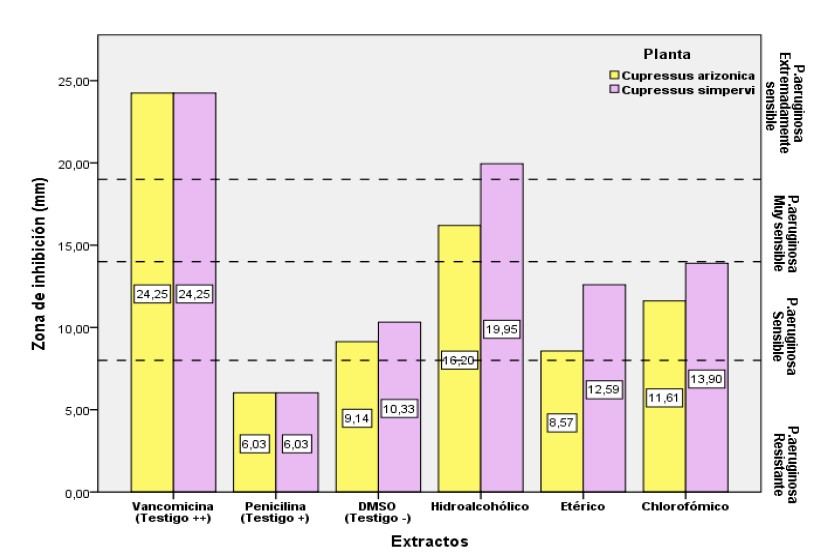
The objective of our research is to identify the chemical components and evaluate in vitro, the antibacterial activity of the extracts from the leaves of Cupressus sempervirens L. and Cupressus arizonica L. from northern Algeria against Pseudomonas aeruginosa ATCC 9027. The extraction was carried out by macerating the leaves in solvents of increasing polarity (chloroform, petroleum ether and aqueous methanol). A screening of the phenolic compounds was carried out for a qualitative characterization of the different extracts. The extracts obtained were put in contact with a strain of P. aeruginosa to determine their antibacterial potential. Phytochemical screening revealed the presence of several secondary metabolites: leucoanthocyanins, flavonols, flavonones, anthraquinones, catechin tannins, gallic tannins, steroids, triterpenes, saponin, cardiac glycosides, terpenoids, saponosides and free quinones. Aqueous methanol (high polarity) allows the extraction of most metabolites. The best extraction yield of the three solvents is chloroform, with an extraction yield of 61.23% (for C. sempervirens) and 52.27% (for C. arizonica), followed by the hydroalcoholic solvent 33.55% and the ethereal solvent with 0.39%. Hydroalcoholic extraction induces a very important sensitivity of P. aeruginosa, with a diameter of 16.2 mm for C. arizonica. Ethereal and chloroformic extracts induce weak inhibition. P. aeruginosa is extremely sensitive to the hydroalcoholic extract from C. sempervirens, the latter induces an inhibition zone with a diameter of 19.95 mm, which is statistically equal to that induced by Vancomycin. These results can be considered as a promising solution for the replacement of vancomycin with the hydroalcoholic extract from C. sempervirens.
Downloads
References
Azzaz, N. A.; Hamed, S. S. and Kenawy, T. A. 2019. Chemical studies on cypress leaves (Cupressus sempervirens) and their activity as antimicrobial agents. Al-Azhar J. Agric. Res. 44(2):100-109.
Benavente-Garcia, O.; Castillo, J.; Marin, F. R.; Ortuno, A. and Del-Rio, J. A. 1997. Uses and properties of citrus flavonoids, J. Agric. Food Chem. 45(12):4505-4515. DOI: https://doi.org/10.1021/jf970373s
Bouayed, J.; Rammal, H.; Younos, C.; Dicko, A. and Soulimani, R. 2008. Caractérisation et bio évaluation des polyphénols: nouveaux domaines d’application en santé et nutrition. Springer. 4(6):71-74. DOI: https://doi.org/10.1007/s10298-008-0292-4
Bruneton, J. 1993. Les tannins. (Ed.). Médicinales internationales. Paris. 404-407 pp.
Bssaibis, F.; Gmira, N. and Meziane, M. 2009. Activité antibactérienne de Dittrichia viscosa (L). W. Greuter. Rev. Microbiol. Ind San Environ. 3:44-55. Celiktas, O.; Hames-Kocabas, E.; Bedir, E.; Sukan, F.; Ozek, T. and Baser, K. 2007. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis L., depending on location and seasonal variations. Food Chem. 100(2):553-9. DOI: https://doi.org/10.1016/j.foodchem.2005.10.011
Chaitra, S.; Kumar, N.; Shalini, P.; Sindhu, R. and Raj, K. 2015. Phytochemical analysis and antibacterial activity of Albergia paniculata roxb. International journal of pharmaceutical sciences and research. 6(2):712-716.
Chaudhary, H.; Shahid, W.; Bano, A.; Ullah, F.; Munis, F.; Fahad, S. and Ahmad, I. 2012. In vitro analysis of Cupressus sempervirens L. plant extracts antibaterial activity. J. Med. Plants Res. 6(2):273-276. DOI: https://doi.org/10.5897/JMPR11.1246
Debib, A.; Tir-touil, A.; Mothana, R.; Meddah, B. and Sonnet, P. 2014. Phenolic content, antioxidant and antimicrobial activities of two fruit varieties of Algerian Ficus carica L. J. Food Bio. 38(2):207-215. DOI: https://doi.org/10.1111/jfbc.12039
Djahra, A. 2014. Etude phytochimique et activité antimicrobienne, antioxydante, antihépatotoxique du Marrube blanc ou Marrubium vulgare L. Universite badji mokhtar annaba. Thèse de doctorat. 114-120 pp.
Evans, W. C. 1996. Phytochemical screening. In: Trease GE, Evans WC, (Ed). Textbook of pharmacognosy. London: tindal limited. 541-548.
Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M. and Abdelly, C. 2008. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. Compt. Rend. Biol. 331(5):372-379. DOI: https://doi.org/10.1016/j.crvi.2008.02.008
Farhat, A.; Ginies, C.; Romdhane, M. and Chemat, F. 2009. Eco-friendly and cleaner process for isolation of essential oil using microwave energy: experimental and theoretical study. J. Chromatogr. A. 1216(26):5077-5085. DOI: https://doi.org/10.1016/j.chroma.2009.04.084
Graglia, E.; Julkunen-Tïito, R. and Shaver, G. 1996. Environmental control and intersite variations of phenolics in Betuna nana in tundra ecosystems. New Phytologist. 151(1):227-236. DOI: https://doi.org/10.1046/j.1469-8137.2001.00149.x
Harborne, A. J. 1998. Phytochemical methods a guide to modern techniques of plant analysis, 3 ème (Ed.). Springer, Netherlands. 302-311.
Harborne, J. B. 1973. Phytochemical methods. London chapman and hall, Ltd. 49-56 pp.
Hayouni, E. A.; Abedrabba, M.; Bouix, M. and Hamdi, M. 2007. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian (Quercus coccifera L.) and (Juniperus phoenicea L.) fruit extracts. Food Chem. 105(3):1126-34. DOI: https://doi.org/10.1016/j.foodchem.2007.02.010
Hiermann, A.; Schramm, H. W. and Laufer, S. 1998. Antiinflammatory activity of myricitin-3-o-beta-D-glucuronide and related compounds. Inflamm. 47(11):421-427. DOI: https://doi.org/10.1007/s000110050355
Hodek, P.; Trefil, P. and Stiborov, A. M. 2002. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 139(1):1-21. DOI: https://doi.org/10.1016/S0009-2797(01)00285-X
Kanwal, Q.; Hussain, I.; Latif-Siddiqui, H. and Javaid, A.2010. Antifungal activity of flavonoids isolated from mango (Mangifera indica L.) leaves. Nat. Prod. Res. 24(20):1907-14. DOI: https://doi.org/10.1080/14786419.2010.488628
Lakhdar, L. 2015. Evaluation de l’activite antibactérienne d’huiles essentielles marocaines sur Aggregatibacter actinomycetemcomitans: étude in vitro. Université mohammed v rabat. Maroc. Thése de doctotrat. 183 p.
Liu, C. M.; Zhou, H. B. and Zhang, W. D. 2010. Terpenoids from stems and leaves of Cupressus gigantea. Chin. J. Nat. Med. 8(6):0405-0410. DOI: https://doi.org/10.1016/S1875-5364(11)60002-2
Lozniewski, A. and Rabaud, C. 2010. Résistance bactérienne aux antibiotiques, Fiches conseils pour la prévention du risque infectieux-Infections associées aux soins, CCLIN, Sud-Est, Nancy. 4- 6 pp.
Macheix, J. J.; Fleuriet, A. and Jay-Allemand, C. 2005. Les composées phénoliques des végétaux. France. (Ed.). Presses Polytechniques. 192 p.
Ngameni, B.; Kuete, V.; Simo, I. K.; Mbaveng, A. T.; Awoussong, P. K.; Patnam, R.; Roy, R. and Ngadjui, B. T. 2009. Antibacterial and antifungal activities of the crude extract and compounds from Dorstenia turbinata (Moraceae). S. Afr. J. Bot. 75(2):256-61. DOI: https://doi.org/10.1016/j.sajb.2008.11.006
Nostro, N.; Germano, M.; Ángelo, V. D. and Cannatelli, M. 2000. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Letters Appl. Microbiol. 30(5):379-384. DOI: https://doi.org/10.1046/j.1472-765x.2000.00731.x
Ponce, A. G.; Fritz, R.; Del-Valle, C. and Roura, S. I. 2003. Antimicrobial activity of essential oils on the native microflora of organic swiss chard. Lwt-Food Sci Technol. 36(7):679-84. DOI: https://doi.org/10.1016/S0023-6438(03)00088-4
Quezel, P. and Santa, S.1963. Nouvelle flore de l’Algérie. Tome I. centre national de la recherche scientifique. Paris, France. 34-35 pp.
Ribéreau-Gayon, P. 1968. Les composés phénoliques des végétaux. (Ed.). Dunod, Paris. 232-242 pp.
Romani, A.; Pinelli, P.; Cantini, C.; Cimato, A. and Heimler, D. 2006. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L). J. Food Chem. 95(2):221-225. DOI: https://doi.org/10.1016/j.foodchem.2005.01.013
Scalbert, A. 2004. Fruits et légumes, polyphénols et santé, laboratoires des maladies métaboliques et micronutritions, INRA, Centre de recherche de clermont-ferrand/theix. 198-203 pp.
Selim, S. A.; Adam, M. E.; Hassan, S. M. and Albalawi, A. R. 2014. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and (Cupressus sempervirens L.). BMC complementary and alternative medicine. 14(179):1-8. DOI: https://doi.org/10.1186/1472-6882-14-179
Shunying, Z.; Yang, Y.; Huaidong, Y.; Yue, Y. and Guolin, Z. 2005. Chemical composition and antibacterial activity of the essential oils of Chrysanthemum indicum. J. Ethnopharmacol. 96(2):151-158. DOI: https://doi.org/10.1016/j.jep.2004.08.031
Trease, G. E. and Evans, W. C. 1989. Pharmacognosy. 27. 13th (Ed.). London: ELBS/Bailliere Tindall, London. 345-772 pp.
Trease, G. E. and Evans, W. C. 2002. Pharmacognosy. 15th (Ed.). Saunders. 214-393 pp.
Zimmer, N. and Cordesse, R. 1996. Influence des tannins sur la valeur nutritive des aliments des ruminants. INRA. Prod Anim. 9(3):167-179.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


