Study on the production of Aspergillus niger proteases using whey
DOI:
https://doi.org/10.29312/remexca.v16i30.4047Keywords:
enzymes, fermentation, peptides, protease, proteinsAbstract
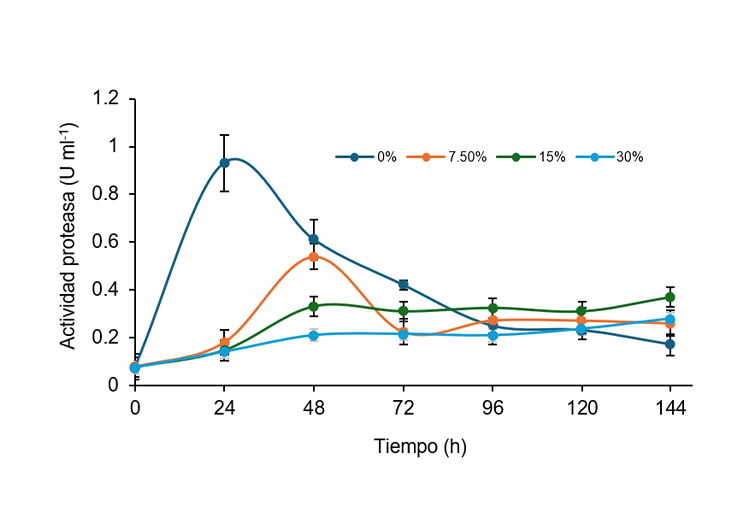
Whey is a byproduct or waste product of the dairy industry, generally of little use, with a high protein content that can induce fungal synthesis of proteolytic enzymes. This study aimed to evaluate the sustainable production of proteolytic enzymes of Aspergillus niger using whey as a substrate in liquid fermentation. Fermentation was carried out in flasks with 50 ml of whey culture medium and glucose concentrations in the Laboratory of Fermentations and Biomolecules in 2024. Fermentation was performed at 30 °C with orbital shaking at 150 rpm for 48 h. The fermentation extracts were analyzed for peptide content and protease activity. The results showed that the enzyme extract without added glucose produced the highest protease activity after 24 h. The use of this extract at a pH of 3.5 resulted in a higher release of peptides from the whey. Whey, being a complex substrate that contains sugars, proteins, fats, and minerals, can influence the growth of microorganisms and the production of enzymes. The valorization of this agro-industrial waste provides an effective and sustainable method to produce biomolecules for agri-food and agro-industrial use.
Downloads
References
AOAC. 1980. Official Methods of Analysis. Association of Official Analytical.
Benítez-Torre, A.; Pérez-Ramírez, E.; Morales-García, Y. E.; Muñoz-Rojas, J.; Díaz-Ruíz, R. y Morales-Almora, P. 2023. Efecto del uso de lactosuero dulce en el riego de alfalfa y maíz. Revista Mexicana de Ciencias Agrícolas. 14(29):1-12. https://doi.org/10.29312/remexca.v14i29.3532.
Boscaini, S.; Cabrera-Rubio, R.; Nychyk, O.; Speakman, J. R.; Cryan, J. F.; Cotter, P. D. and Nilaweera, K. N. 2020. Physiological Reports. 8(15):1-19. https://doi.org/10.14814/phy2.14523.
Cairns, T. C.; Nai, D. O. and Meyer, V. 2018. Cómo un hongo influye en la biotecnología: 100 años de investigación sobre Aspergillus niger. Biología y biotecnología de los hongos. 5(13):1-14. https://doi.org/10.1186/s40694-018-0054-5.
Cho, K.; Lee, J.; Han, G.; Kim, N. K.; Bae, H. and Hwang, S. 2015. Resource recovery using whey permeate to cultivate Phellinus linteus mycelium: Solid-state and submerged liquid fermentation. Journal of Dairy Science. 98(10):6739-6748. https://doi.org/10.3168/jds.2015-9631.
Crament, T. C.; Arendsen, K.; Rose, S. H. and Jansen, T. 2024. Cultivation of recombinant Aspergillus niger strains on dairy whey as a carbohydrate source. Journal of Industrial Microbiology and Biotechnology. 9(51):1-7. Doi:10.1093/jimb/kuae007.
Cen-Li; Jingwen-Zhou; Guocheng-Du; Jian-Chen; Shunji-Takahashi and Song-Liu. 2020. Developing Aspergillus niger as a cell factory for food enzyme production. Biotechnology Advances. 44:1-17. https://doi.org/10.1016/j.biotechadv.2020.107630.
Dinika, I. and Utama, G. L. 2019. Cheese Whey is a potential resource for antimicrobial edible film and active packing production. Foods and Raw Materials. 7(2):229-239. http://doi.org/10.21603/2308-4057-2019-2-229-239.
DuBois, M.; Gilles, K. A.; Hamilton, J. K.; Robers, P. A. and Smith, F. 1956. Colorimetric method for the determination of sugars and related substances. Analytical Biochemistry. 28(3):350-356. Doi: 10.1021/ac60111a017.
Fernández-Gutiérrez, D.; Veillette, M. and Giroir-Fendler, A. 2017. Biovalorization of saccharides derived from industrial wastes such as whey: a review. Reviews in Environmental Science Biotechnology. 16(1):147-174. https://doi.org/10.1007/s11157-016-9417-7.
Gill, H. S.; Doull, F.; Rutherfurd, K. J. and Cross, M. L. 2000. Immunoregulatory peptides in bovine milk. British Journal of Nutrition. 84(S1):111-117. https://doi.org/10.1017/s0007114500002336.
Irazoqui, J. M.; Santiago, G. M.; Mainez, M. E.; Amadio, A. F. and Eberhardt, M. F. 2024. Enzymes for production of whey protein hydrolysates and other value-added products. Applied Microbiology and Biotechnology. 108(1):1-11. https://doi.org/10.1007/s00253-024-13117-2.
John, J. A. and Ghosh, B. C. 2021. Production of whey protein hydrolyzates and its incorporation into milk. Food Production Processing and Nutrition. 3(1):1-13. https://doi.org/10.1186/s43014-021-00055-z.
Johnvesly, B.; Manjunath, B. and Naik, G. R. 2002. Pigeon pea waste as a novel, inexpensive, substrate for production of a thermostable alkaline from a thermoalkalophilic Bacillus sp. JB-99. Bioresource Technology. 82(1):61-64. https://doi.org/10.1016/S0960-8524(01)00147-X.
Kashung, P. and Karuthapandian, D. 2025. Milk-derived bioactive peptides. Food Production Processing and Nutrition. 7(1):1-19. https://doi.org/10.1186/s43014-024-00280-2.
Kunitz, M. 1947. Crystalline soybean tripsin inhibitor: ii. General properties. Journal of general physiology. 30(4):291-310. https://doi.org/10.1085/jgp.30.4.291.
Lam, F.; Khan, T. M.; Faidah, H.; Haseeb, A. and Khan, A. H. 2019. Effectiveness of whey protein supplements on the serum levels of amino acid, creatinine kinase and myoglobin of athletes: a systematic review and meta-analysis. Systematic Reviews. 8(1):1-12. https://doi.org/10.1186/s13643-019-1039-z.
Li, W.; Wang, J.; Zhang, J.; Takada, S. and Wang, W. 2019. Overcoming the Bottleneck of the enzymatic cycle by steric frustration. Physical Review Letters. 122(23):1-6. https://doi.org/10.1103/physrevlett.122.238102.
Li, J.; Chroumpi, T.; Garrigues, S.; Kun, R. S.; Meng, J.; Salazar-Cerezo, S.; Aguilar-Pontes, M. V.; Zhang, Y.; Tejomurthula, S.; Lipzen, A.; Ng, V.; Clendinen, C. S.; Tolić, N.; Grigoriev, I. V.; Tsang, A.; Mäkelä, M. R.; Snel, B.; Peng, M. and Vries, R. P. 2022. The sugar metabolic model of Aspergillus niger can only be reliably transferred to fungi of its phylum. Journal of Fungi. 8(12):1-23. https://doi.org/10.3390/jof8121315.
Liu, X.; Lian, M.; Zhao, M. and Huang, M. 2024. Advances in recombinant protease production: current state and perspectives. World Journal of Microbiology and Biotechnology. 40(5):144. https://doi.org/10.1007/s11274-024-03957-5.
Mordor Intelligence. 2025. Protease market size and share analysis growth trends and forecasts source. https://www.mordorintelligence.com/industry-reports/proteases-market.
Norma Oficial Mexicana. 2012. NOM-155-SCFI-2012, Leche denominaciones, especificaciones fisicoquímicas, información comercial y métodos de prueba, secretaria de economía. Estados Unidos Mexicanos. http://sidof.segob.gob.mx/notas/5254842.
Naveed, M.; Nadeem, F.; Mehmood, T.; Bilal, M.; Anwar, Z. and Amjad, F. 2021. Protease a versatile and ecofriendly biocatalyst with multi-industrial applications: an updated review. Catalysis Letters. 151(2):307-323. https://doi.org/10.1007/s10562-020-03316-7.
Nouri, N.; Sadeghi, L. and Marefat, A. 2024. Production of alkaline protease by Aspergillus niger in a new combinational paper waste culture medium. Journal of bioscience and bioengineering. 137(3):173-178. https://doi:10.1016/j.jbiosc.2023.12.010.
Quintieri, L.; Luparelli, A.; Caputo, L.; Schirinzi, W.; Bellis, F.; Smiriglia, L. and Monaci, L. 2025. Unraveling the biological properties of whey peptides and their role as emerging therapeutics in immune tolerance. Nutrients. 17(6):1-29. https://doi.org/10.3390/nu17060938.
Rojas, M. P. G. and Martinez, O. M. M. 2023. Whey protein fermentation with Aspergillus niger: source of antioxidant peptides. En Mehdi Razzaghi-Abyaneh, Mahendra Rai y Masoomeh Shams-Ghahfarokhi (Eds.). Aspergillus and aspergillosis - Advances in genomics, drug development, diagnosis and treatment. (IntechOpen, 1st ed, 208 p.). https://doi.org/10.5772/intechopen.111895.
Siala, R.; Frikha, F.; Mhamdi, S.; Nasri, M. and Kamoun, A. S. 2012. Optimization of acid protease production by Aspergillus niger I1 on shrimp peptone using statistical experimental design. The Scientific World Journal. 1-11 pp. https://doi.org/10.1100/2012/564932.
Tang, C.; Xi, T.; Zheng, J. and Cui, X. 2025. Chemical properties of whey protein in protein powders and its impact on muscle growth in athletes: a review. Natural Product Communications. 20(3):1-7. https://doi.org/10.1177/1934578x251326124.
Wang, R.; He, S. and Xuan, Y. 2020. Preparation and characterization of whey protein hydrolysate-Zn complexes. Food Measure. 14:254-261. https://doi.org/10.1007/s11694-019-00287-1.
Yin, X.; Fu, X.; Cheng, H. and Wusigale-Liang, L. 2020. α-Tocopherol and naringenin in whey protein isolate particles: partition, antioxidant activity, stability and bioaccessibility. Food Hydrocolloids. 106:105895. https://doi.org/10.1016/j.foodhyd.2020.105895.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


