Comparison of organically derived IAA and synthetic IAA in rosa Forever cuttings
DOI:
https://doi.org/10.29312/remexca.v16i5.3771Keywords:
Pseudomona guariconensis, auxins, vegetative propagation, strainAbstract
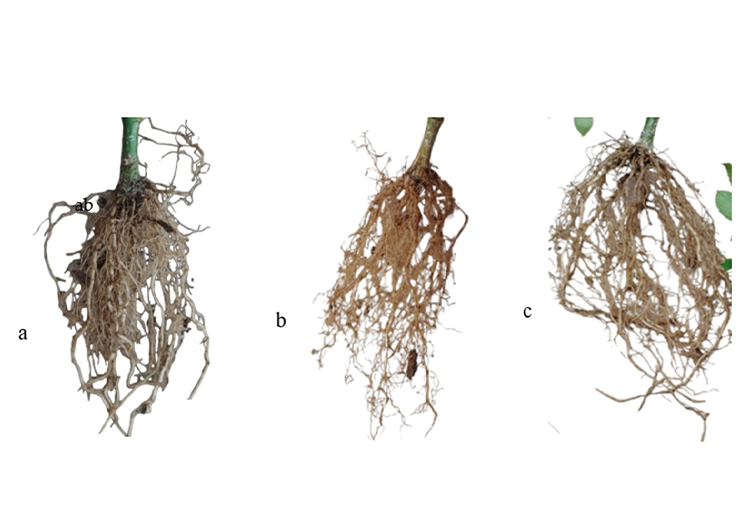
The genus Rosa sp. Forever Young, belonging to the rose family, is the most important crop in the ornamental sector, which represents one of the main products in the floriculture market. The propagation of roses is usually asexual and is done by cuttings or grafts. Auxins, such as indole acetic acid, stimulate root formation and increase the percentage of rooting of cuttings and survival, so they are used to improve propagation. The research aimed to determine the effect on vegetative propagation of cuttings of Rosa sp. Forever Young with synthetic indole acetic acid and with organically derived indole acetic acid present in supernatants from culture of the strain of Pseudomonas guariconensis RMC6. Cuttings of approximately 10 cm were obtained and immersed for 3 h in each treatment. The research was conducted in a completely randomized experimental design, with eight treatments, with four replications. The experimental unit was a pot with 25 cuttings per pot, having a total of 100 cuttings. It was observed that the interaction given by the inoculation between the cuttings of rosa Forever and the organically derived indole acetic acid present in the culture supernatants of the strain had a positive effect on the variables of root length, root diameter, root number, stem diameter, and number of shoots; the replacement of synthetic auxin with the organically derived auxin from the strain of P. guariconensis RMC6 can be suggested.
Downloads
References
Bordiec, S.; Paquis, S.; Lacroix, H.; Dhondt, S.; Barka, E.; Kauffmann, S.; Jeandet, P.; Mazeyrat-Gourbeyre, F.; Clement, C.; Baillieul, F.; and Dorey, S. 2011. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas Syringae pv. Pisi in grapevine cell suspensions. Journal of Experimental Botany. 62(2):595-603. https://doi.org/10.1093/jxb/erq291.
Cordero, P.; Príncipe, A.; Jofré, E.; Mori, G.; and Fischer, S. 2014. Inhibition of the phytopathogenic fungus Fusarium proliferatum by volatile compounds produced by Pseudomonas. Arch. Microbiol. 196(11):803-809. Doi:10.1007/s00203-014-1019-6.
De la Cadena-Vera, J. E. 2005. Efectos de la inoculación con micorriza vesículo-arbuscular en la producción de rosas en Pichincha, Ecuador. Honduras. 28 p.
Díaz, K.; Valiente, C.; Martínez, M.; Castillo, M. and Sanfuentes, E. 2009. Root-promoting rhizobacteria in Eucalyptus globulus cuttings. World J Microbial Biotechnol. 25:867-873. Doi:10.1007/s11274-009-9961-1.
Felker, P.; Medina, D.; Soulier, C.; Velicce, G.; Velarde, M. and González, C. 2005. A survey of environmental and biological factors (Azospirillum spp., Agrobacterium rhizogenes, Pseudomonas aurantiaca) for their influence in rooting cuttings of Prosopis alba clones. Journal of Arid Environments. 61(2):227-247. Doi: 10.1016/j.jaridenv.2004.09.010.
Fernández, A. M. 2014. Técnicas tradicionales y biotecnológicas en mejoramiento genético de la rosa. Universidad Autónoma del Estado de México (UAEM). Toluca, Estado de México. 18 p.
García-Rubio, L. A.; Vargas-Ponce, O.; Ramírez-Mireles, F. J.; Munguía-Lino, G.; Corona-Oceguera, C. A. y Cruz-Hernández, T. 2015. Distribución Geográfica de Hylocereus (Cactaceae) en México. Botanical Sciences 93(4):921-939. Doi:10.17129/botsci.282.
González-Candia, P.; Rodríguez, F.; Sanfuentes, V. S. E. A. y Sossa, F. K. 2016. efecto de rizobacterias en el enraizamiento de miniestacas en dos clones híbridos de Eucalyptus spp. Ciencia e Investigación Forestal INFOR. 22(1):51-63. https://doi.org/10.52904/0718-4646.2016.450.
Grossmann, K. 2010. Auxin herbicides: status of mechanism and mode of action. Pest Manage Sci. 66(2):113-120. https://doi.org/10.1002/ps.1860.
Kamilova, F.; Kravchenko, L. V.; Shaposhnikov, A. I.; Azarova, T.; Makarova, N. X. and Lugtenberg, B. J. J. 2006. Organic acids, sugars and L-tryptophane in exudates of vegetables growing on Stonewood and their effects on activities of rhizosphere bacteria. Mol. Plant Microbe Interact. 19(3):250-56. https://doi.org/10.1094/MPMI-19-0250.
Kashefi, M.; Zarei, H. and Bahadori, F. 2014. The regulating effect of the growth of indole butyric acid and the time of stem cutting preparation on peroliferation of Damask rose ornamental Shrubjournal of ornamental plants. 4(4):49-55.
Kaymak, H. C.; Yarali, F.; Guvenc, I. and Donmez, M. F. 2008. The effect of inoculation with plant growth rhizobacteria (PGPR) on root formation of mint (Mentha piperita L.) cuttings. African Journal of Biotechnology. 7(24):4479-4483.
Khan, M. S.; Khan R. U. and Waseem, K. 2006. Effect of some auxins on growth of damask rose cuttings in different growing media. J. Agric. Soc. Sci. 2(1):13-16 http://www.fspublishers.org.
Kloepper, J. W.; Ryu, C. M. and Zhang, S. 2004. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 94(11):1259-66. https://doi.org/10.1094/PHYTO.2004.94.11.1259.
Leveau, J. H. and Lindow, S. E. 2005. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl. Environ. Microbiol. 71(5):2365-71. https://link.springer.com/article/10.1023/B:MICI.0000023982.76684.9d.
Luziatelli, F.; Grazia, F. A.; Bonini, P.; Muleo, R.; Gatti, L.; Meneghini, M.; Tronati, M.; Melini, F. y Ruzzi, M. 2020. Una perspectiva genética y metabolómica sobre la producción de ácido indol-3-acético por Pantoea agglomerans y el uso de sus metabolitos como bioestimulantes en viveros de plantas. 11:1-17 Doi: https://doi.org/10.3389/fmicb.2020.01475.
Márquez, L. S.; Huacán, V. R. E. and Huarhua, C. T. 2018. Efecto de tres enraizadores y dos tipos de sustratos en estacas de rosa (Rosa sp.) del patrón natal Brier en condiciones de Vivero en el Instituto de Educación Rural (IER) San Salvador, Calca-Cusco. Revista Ciencia y Tecnología para el Desarrollo-UJCM. 4(7):22-28. https://hdl.handle.net/20.500.12819/211.
Montero-Calasanz, M. C.; Santamaría, C.; Albareda, M.; Daza, A.; Duan, J.; Glick, B. R. and Camacho, M. 2013. Alternative rooting induction of semi-hardwood olive cuttings by several auxin-producing bacteria for organic agriculture systems. Spanish Journal of Agricultural Research. 11(1):146-154. http://dx.doi.org/10.5424/sjar/2013111-2686.
Patten, C. and Glick, B. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Applied and Environmental Microbiology. 68(8):3795-3801. https://doi.org/10.1128/AEM.68.8.3795-3801.2002.
Quispe, A. 2017. Adaptación y rendimiento de 20 clones de camote (Ipomoea batatas L.) de doble propósito en el ecosistema de Bosque Seco, Piura. Ciencia y Desarrollo. 20(1):15-48. Doi:http://dx.doi.org/10.21503/cyd.v20i1.1407.
Salantur, A.; Ozturk A.; Akten, S.; Sahin, F. and Donmez, F. 2005. Effect of inoculation with non-indigenous and indigenous Rhizobacteria of Erzurum (Turkey) origin on growth and yield of spring barley. Plant Soil. 275(1):147-156. https://link.springer.com/article/10.1007/s11104-005-8094-z.
Sánchez, C. R. y Guerra, R. P. 2022. Pseudomonas spp. benéficas en la agricultura Revista Mexicana de Ciencias Agrícolas. 13(4):715-725. doi.org/10.29312/remexca.v13i4.2799.
Sezen, I.; Kaymak, H. Ç.; Aytatli, B.; Dönmez, M. F. and Ercişli, S. 2014. Inoculations with plant growth promoting rhizobacteria (PGPR) stimulate adventitious root formation on semi-hardwood stem cuttings of Ficus benjamina. Prop. Orn. Plants. 14(4):152-157.
SIAP. 2020. https://nube.siap.gob.mx/gobmx-publicaciones-siap/pag/2020/Atlas.
Sitinjak, R. R. 2015. The growth response stem cuttings of roses (Rosa sp.) to plant growth regulator Atonik and Rootone F. Journal of Chemical and Pharmaceutical Research. 7(9):557-562 https://www.researchgate.net/profile/Rama-Sitinjak/publication/295980263.
Spaepen, S.; Vanderleyden, J. and Remans, R. 2007. Indole-3-acetic acid in microbial and microorganism-plant signaling. In: unden F. Ed FEMS microbiol rev. Blackwell Publishing Ltd., New York. 1-24 pp. Doi:10.1111/j.1574-6976.2007.00072.x.
Tariq, U.; Riaz, A.; Jaskani, M. J. and Zahir, Z. A. 2016. Screening of PGPR isolates for plant growth promotion of Rosa damascena. Int. J. Agric. Biol. 18(5):997-1003. Doi:10.17957/IJAB/15.0200.
Tsavkelova, E. A.; Cherdyntseva, T. A.; Botina, S. G. and Netrusov, A. I. 2007. Bacteria associated with orchid roots and microbial production of auxin. Microbiological Research. 162(1):69-76. Doi: 10.1016/j. micres.2006.07.014.
Ulcuango, C. O. 2019. Evaluación de la propagación de rosa (Rosa spp.) por estacas mediante el uso de ácido naftalenacético en el Cantón Pedro. 1 p. http://repositorio.utn.edu.ec/handle/123456789/9228.
Vega-Celedón, C.; González. M. y Seeger, M. H. 2016. Biosíntesis de ácido indol-3-acético y promoción del crecimiento de plantas por bacterias. Cultivos Tropicales. 37(esp):33-39. https://www.redalyc.org/journal/1932/193246189005/htm.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


