Mutagenesis by gamma radiation for genetic improvement of food-important plants
DOI:
https://doi.org/10.29312/remexca.v15i5.3747Keywords:
ionizing radiation, plant breeding, plant tissue cultureAbstract
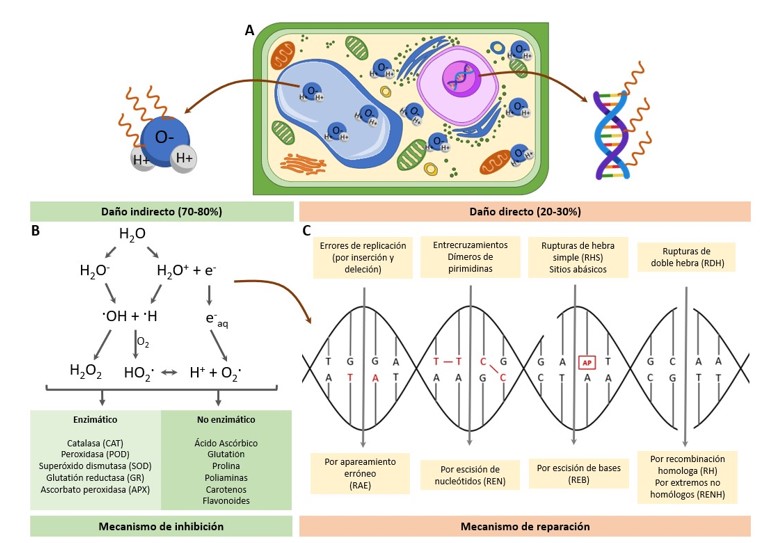
Mutagenesis induced by physical agents such as Co60 gamma radiation in plant cells or tissues generates structural changes in deoxyribonucleic acid and has increased genetic variability in crops of agricultural importance. Often, the starting material is plant species established in in vitro cultures, which facilitates the management and control of physicochemical conditions in addition to increasing the number of repetitions in a minimum space. As a product, it is expected to obtain improved varieties with tolerance to biotic or abiotic factors in addition to improving morphological and nutritional qualities. This review of the art study compiled information from the last 10 years to provide a current overview of the effect of gamma radiation on plant tissues in vitro, addressing from radiation sources, types of damage and repair mechanisms of deoxyribonucleic acid, in addition to the use of molecular markers to evidence variations at the genetic level. Success cases for crops of agro-industrial importance in Mexico will be analyzed, sharing the current expectations in the use of this technology.
Downloads
References
Abdelnour-Esquivel, A.; Pérez, J.; Rojas, M.; Vargas, W. y Gatica-Arias, A. 2020. Use of gamma radiation to induce mutations in rice (Oryza sativa L.) and the selection of lines with tolerance to salinity and drought. In vitro cellular and developmental biology plant. 56:88-97. https://doi.org/10.1007/s11627-019-10015-5.
Ali, H.; Ghori Z.; Sheikh, S. and Gul, A. 2015. Effects of gamma radiation on crop production. In: crop production and global environmental issues. Hakeem, K. Ed. Springer Cham. Switzerland. 27-78 pp.
Ángeles-Espino, A.; Dimas-Estrada, H. E.; Ramírez-Alvarado, D.; Cruz-Rubio, J. M.; Palmeros-Suárez, P. A. y Gómez-Leyva, J. F. 2020. Caracterización molecular de mutantes de Agave tequilana inducidas con radiación gamma Co60 y su efecto en la acumulación de fructooligosacáridos. Acta Universitaria. 30:1-11. https://doi.org/10.15174/au.2020.2696.
Bali, B. K. 2023. Different Types of DNA molecular markers and genotyping applications for mutation breeding. in biotechnologies and genetics in plant mutation breeding. Ahmad, Ed. Revolutionizing Plant Biology. 2086-2091 pp. https://doi.org/10.1201/9781003305088.
Bhat, R. S.; Brijesh, M. P.; Tilak, I. S. and Shirasawa, K. P. 2023. Molecular markers for mutant characterization. Ed. In: mutation breeding for sustainable food production and climate resilience. Springer Nature. Singapure. 205-232 pp.
Demidchik, V. 2015. Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environmental and Experimental Botany. 109:212-228. http://dx.doi.org/10.1016/j.envexpbot.2014.06.021.
Due, M. S.; Susilowati, A. and Yunus, A. 2019. The effect of gamma rays irradiation on diversity of Musa paradisiaca var. sapientum as revealed by ISSR molecular marker. Biodiversitas journal of biological diversity. 20(5):1416-1422. https://doi.org/10.13057/biodiv/d200534.
El-Fiki, A.; Fahmy, E.; Doma, A. A.; Helmy, O.; Adly, M. y El-Metabteb, G. 2021. The genetic variation assessment of in vitro irradiated tomato (Lycopersicon esculentum Mill) by SCoT and ISSR markers. Journal of microbiology, biotechnology and food sciences. 10(4):557-565. https://doi/10.15414/jmbfs.2021.10.4.557-565.
Hong, M. J.; Kim, J. B.; Yoon, Y. H.; Ki, S. H.; Ahn, J. W.; Jeong, I. Y. and Kim, D. S. 2014. The effects of chronic gamma irradiation on oxidative stress response and the expression of anthocyanin biosynthesis-related genes in wheat (Triticum aestivum). International journal of radiation biology. 90(12):1218-1228. https://doi.org/10.3109/09553002.2014.934930.
Huerta-Olalde. A. M; Hernández-García A.; López-Gómez. R.; Fernández-Pavía, S. P.; Zavala-Páramo, M. G. and Salgado-Garciglia R. 2022. In vitro selection of blackberry (Rubus fruticosus ‘Tupy’) plants resistant to Botrytis cinerea using gamma ray-irradiated shoot tips. Plant Biotechnology. 39(2):165-171. https://doi.org/10.5511/plantbiotechnology.22.0312b.
IAEA. 2022b. Organización internacional de energía atómica. Base de datos de variedades mutantes. https://nucleus.iaea.org/sites/mvd/SitePages/Search.aspx.
Kariuki, J.; Horemans, N.; Saenen, E.; Hees, M.; Verhoeven, M.; Nauts, R.; Gompel A. V.; and Cuypers, A. 2019. The responses and recovery after gamma irradiation are highly dependent on leaf age at the time of exposure in rice (Oryza sativa L.). Environmental and experimental botany. 162:157-167. https://doi.org/10.1016/j.envexpbot.2019.02.020.
Le, K. C.; Ho, T. T.; Paek, K. Y. and Park, S. Y. 2019. Low dose gamma radiation increases the biomass and ginsenoside content of callus and adventitious root cultures of wild ginseng (Panax ginseng Mayer). Industrial crops and products. 130(408):6-24. https://doi.org/10.1016/j.indcrop.2018.12.056.
Ludovici, G. M.; de Souza, S. O.; Chierici, A.; Cascone, M. G.; Errico, F. and Malizia, A. 2020. Adaptation to ionizing radiation of higher plants: From environmental radioactivity to chernobyl disaster. Journal of environmental radioactivity. 222:1-10. https://doi.org/10.1016/j.jenvrad.2020.106375.
Manova, V. and Gruszka, D. 2015. DNA damage and repair in plants from models to crops. Frontiers in plant science. 6:1-26. https://doi.org/10.3389/fpls.2015.00885.
Mba, C. 2013. Induced mutations unleash the potentials of plant genetic resources for food and agriculture. Agronomy. 3(1):200-231. https://doi.org/10.3390/agronomy3010200.
Nadeem, M. A.; Nawaz, M. A.; Shahid, M. Q.; Doğanm Y.; Comertpay, G.; Yıldız, M.; Hatipoğlu, R.; Ahmad, F.; Alsaleh, A.; Labhane, N.; Özkan, H. and Baloch, F. S. 2018. DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnology and Biotechnological Equipment. 32(2):261-285. https://doi.org/10.1080/13102818.2017.1400401.
Nikam, A. A.; Devarumath, R. M.; Ahuja, A.; Babu, H.; Shitole, M. G. and Suprasanna, P. 2015. Radiation-induced in vitro mutagenesis system for salt tolerance and other agronomic characters in sugarcane (Saccharum officinarum L.). The crop journal. 3(1):46-56. https://doi.org/10.1016/j.cj.2014.09.002.
Oladosu, Y.; Rafii, M. Y.; Abdullah, N.; Hussin, G.; Ramli, A.; Rahim, H. A.; Miah, G. and Usman, M. 2016. Principle and application of plant mutagenesis in crop improvement: a review. Biotechnology and biotechnological equipment. 30(1):1-16. https://doi.org/10.1080/13102818.2015.1087333.
Penna, S. and Bhagwat, S. G. 2023. Mutagenesis and selection: reflections on the in vivo and in vitro approaches for mutant development. Ed. In: mutation breeding for sustainable food production and climate resilience. Springer nature. Singapure. 99-127 pp. https://doi.org/10.1007/978-981-16-9720-3-1.
Pérez-Jiménez, M.; Ignacio, T. C. and Pérez-Tornero, O. 2020. Inducing mutations in Citrus spp.: Sensitivity of different sources of plant material to gamma radiation. Applied Radiation and Isotopes. 157(13):1-10. https://doi.org/10.1016/j.apradiso.2019.109030.
Puerta-Ortiz, J. A. y Morales-Aramburo, J. 2020. Efectos biológicos de las radiaciones ionizantes. Revista Colombiana de Cardiología. 27(S1):61-71. https://doi.org/10.1016/j.rccar.2020.01.005.
Qi, W.; Zhang, L.; Wang, L.; Xu, H.; Jin, Q. and Jiao, Z. 2015. Pretreatment with low-dose gamma irradiation enhances tolerance to the stress of cadmium and lead in Arabidopsis thaliana seedlings. Ecotoxicology and environmental safety. 115:243-249. https://doi.org/10.1016/j.ecoenv.2015.02.026
Rivai, R. R.; Isnaini, Y. and Yuzammi. A. 2021. Elucidation of the radiosensitivity level of Amorphophallus paeoniifolius (Dennst.) Nicolson embryogenic callus induced by gamma ray irradiation. In Biology and Life Sciences Forum. 11(1):1-8. https://doi.org/10.3390/IECPS2021-11951.
Riviello-Flores M. L.; Cadena-Iñiguez, J.; Ruiz-Posadas L. M.; Arévalo-Galarza, M. L.; Castillo-Juárez, I.; Soto-Hernández, M. and Castillo-Martinez, C. R. 2022. Use of gamma radiation for the genetic improvement of underutilized plant varieties. Plants. 11(9):1-19. https://doi.org/10.3390/ plants11091161.
Royani, J. I.; Abdullah, L. and Aisyah, S. I. 2021. Radio sensitivity of irradiated seed, plantlets, callus, and in vitro leaves from Indigofera zollingeriana Miq by gamma rays. In IOP conference series. Earth and Environmental Science. 913(1):1-10. https://doi.org/10.1088/1755-1315/913/1/012061.
Sharma, V.; Thakur, M. and Tomar, M. 2020. In vitro selection of gamma irradiated shoots of ginger (Zingiber officinale Rosc.) against Fusarium oxysporum f. sp. zingiberi and molecular analysis of the resistant plants. Plant cell, tissue and organ culture (PCTOC). 143(2):319-330. https://doi.org/10.1007/s11240-020-01919-x.
Sharma, V. and Thakur, M. 2021. Gamma irradiations induced morphological and biochemical variations in in vitro regenerated ginger (Zingiber officinale Rosc.) an invaluable medicinal spice. International journal of radiation biology. 97(12):1696-1704. https://doi.org/10.1080/09553002.2021.1988179.
Spencer, M. M.; Forster, B. P. y Jankuloski, L. 2021. Manual de mejoramiento por mutación. FAO/OIEA. 3ra Ed. Viena. https://doi.org/10.4060/i9285es. 5-141 pp.
Szwent, G. A. 2015. Redox chemistry: the essential. Ed. Free radicals in biology and medicine. Oxford university press, USA. 30-77 pp.
Tafurt, Y. C. y Marin, M. A. 2014. Principales mecanismos de reparación de daños en la molécula de DNA. Biosalud. 13(2):95-110.
Udage, A. C. 2021. Introduction to plant mutation breeding: different approaches and mutagenic agents. The journals of agricultural Sciences sri lanka. 16(3):466-483. http://doi.org/10.4038/jas.v16i03.9472.
Yasmin, K.; Arulbalachandran, D.; Soundarya, V. and Vanmathi, S. 2019. Effects of gamma radiation (γ) on biochemical and antioxidant properties in black gram (Vigna mungo L. Hepper). International Journal of Radiation Biology. 95(8):1135-1143. https://doi.org/10.1080/09553002.2019.1589022.
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


