Influence of light on callus generation and in vitro plant culture
DOI:
https://doi.org/10.29312/remexca.v13i27.3156Keywords:
callogenesis, light intensities, organogenesisAbstract
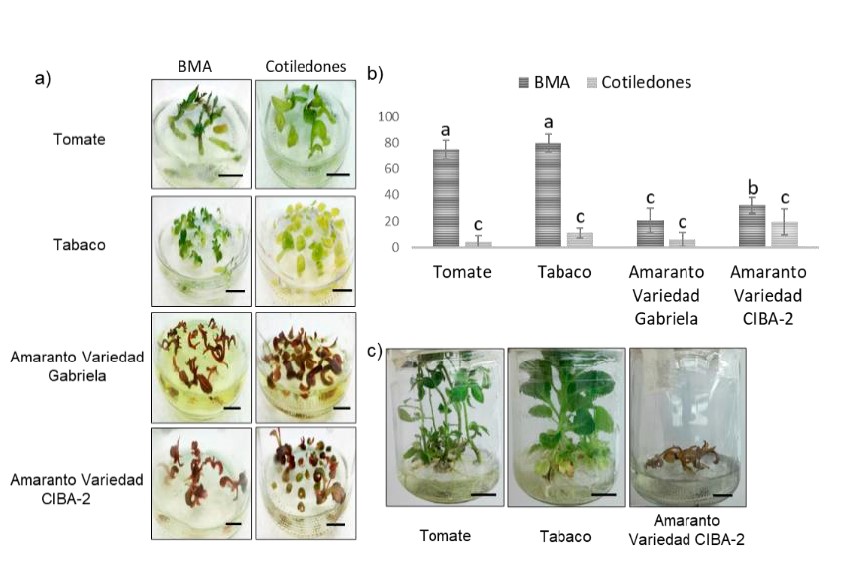
In vitro culture systems are important in the field of plant biotechnology. It has been observed that some factors, including light, affect tissue development under in vitro culture conditions. The objective of this work is to know the effect of light intensity on the development of tissues from seeds under in vitro culture conditions for the generation of seedlings and calluses, in order to favor the times and the regeneration of species of agronomic interest for their comprehensive use. In this work, different light intensities were tested, it was possible to obtain seedlings with calluses, demonstrating that the intensity of light influences the type and development of dedifferentiated structures and that this effect varies inter and intraspecies. By regenerating plant tissues from seedlings, it was found that apical meristem shoots are the most efficient type of explant for clonal regeneration of tobacco and tomato plants. This study is the first to present the effect of light intensity on seeds for the production of seedlings and calluses in different plant species, including a combination of different tissues and explants that could be used to obtain different plant structures for biotechnological purposes.
Downloads
References
Afshari, R. T.; Angoshtari, R. and Kalantari, S. 2011. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics J. 4(2):6067. https://doi.org/10.3316/informit.873216546192644.
Arya, I. D.; Chakravarty, T. N. and Sopory, S. K. 1993. Development of secondary inflorescences and in vitro plantlets from inflorescence cultures of Amaranthus paniculatus. Plant Cell Reports. 12(5):286–288. https://doi.org/10.1007/BF00237137. DOI: https://doi.org/10.1007/BF00237137
Bennici, A.; Grifoni, T.; Schiff, S. and Bovelli, R. 1997. Studies on callus growth and morphogenesis in several species and lines of Amaranthus. Plant Cell, Tissue and Organ Culture. 49(1):29-33. https://doi.org/10.1023/A:1005882322044. DOI: https://doi.org/10.1023/A:1005882322044
Bhatia, P.; Ashwath, N.; Senaratna, T. and Midmore, D. 2004. Tissue culture studies of tomato (Lycopersicon esculentum). Plant Cell, Tissue and Organ Culture. 78(1):1-21. https://doi.org/10.1023/B:TICU.0000020430.08558.6e. DOI: https://doi.org/10.1023/B:TICU.0000020430.08558.6e
Bian, Z. H.; Yang, Q. C. and Liu, W. K. 2015. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review. J. Sci. Food Agric. 95(5):869-877. https://doi.org/10.1002/jsfa.6789. DOI: https://doi.org/10.1002/jsfa.6789
Casal, J. J.; Fankhauser, C.; Coupland, G. and Blázquez, M. A. 2004. Signalling for developmental plasticity. Trends in Plant Sci. 9(6):309-314. https://doi.org/10.1016/j.tplants.2004.04.007. DOI: https://doi.org/10.1016/j.tplants.2004.04.007
Chen, L. L.; Zhang, K.;Gong, X.; Wang, H. Y.; Gao, Y. H.; Wang, X. Q.; Zeng, Z. H. and Hu, Y. G. 2020. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integrative Agric. 19(1):108-119. https://doi.org/ 10.1016/S2095-3119(19)62633-X.
Comia-Yebron, R.; Aspuria, E. T. and Bernardo, E. L. 2017. Callus induction in Amaranthus tricolor and Amaranthus spinosus. J.Inter. Soc. Southeast Asian Agric. Sci. 23(1):12-23.
Espinosa-Leal, C. A.; Puente-Garza, C. A. and García-Lara, S. 2018. In vitro plant tissue culture: means for production of biological active compounds. Planta. 248(2018):1.18. https://doi.org/10.1007/s00425-018-2910-1. DOI: https://doi.org/10.1007/s00425-018-2910-1
Gajdošová, A.; Libiaková, G.; Iliev, I. and Hricová, A. 2013. Adventitious shoots induction of Amaranthus cruentus L. in vitro. Propagation of Ornamental Plants. 13(1):33-49.
Galvão, V. C. and Fankhauser C. 2015. Sensing the light environment in plants: photoreceptors and early signaling steps. Current Opinion in Neurobiology. 34:46-53. https://doi.org/10.1016/j.conb.2015.01.013. DOI: https://doi.org/10.1016/j.conb.2015.01.013
Ganapathi, T. R.; Suprasanna, P.; Rao, P. S. and Bapat, V. A. 2004. Tobacco (Nicotiana tabacum L.) A model system for tissue culture interventions and genetic engineering. Indian J. Biotechnol. 3(2004):171-184.
Gourguillon, L.; Rustenholz, C.; Lobstein, A. and Gondet, L. 2018. Callus induction and establishment of cell suspension cultures of the halophyte Armeria maritima (Mill.) willd. Sc. Hortic. 233(2018):407-411. https://doi.org/10.1016/j.scienta.2017.08.001. DOI: https://doi.org/10.1016/j.scienta.2017.08.001
Huan, L. and Tanaka, M. 2004. Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ. Control Biol. 42(1):57.64. https://doi.org/10.2525/ecb1963.42.57. DOI: https://doi.org/10.2525/ecb1963.42.57
Jamous, F. and Abu-Qaoud, H. 2015. In vitro regeneration of tomato (Lycopersicon esculentum Mill). Plant Cell Biotechnol. Mol. Biol.16(3-4):181-190.
Jehan, S. and Hassanein, A. 2013. Hormonal requirements trigger different organogenic pathways on tomato nodal explants. Am. J.Plant Sci. 4(11):2118-2125. https://doi.org/10.4236/ ajps.2013.411263. DOI: https://doi.org/10.4236/ajps.2013.411263
Khuong, T. T. H.; Crété, P.; Robaglia, C. and Caffarri, S. 2013. Optimisation of tomato Micro-tom regeneration and selection on glufosinate/basta and dependency of gene silencing on transgene copy number. Plant Cell Reports. 32(9):1441-1454. https://doi.org/10.1007/ s00299-013-1456-8. DOI: https://doi.org/10.1007/s00299-013-1456-8
Kumar, N. and Reddy, M. P. 2011. In vitro plant propagation: a review. J. Forest Sci. 27(2):61-72.
Kumar, P. and Dube, S. D. 1997. In vitro plant regeneration from shoot tips of Amaranthus hypochondriacus. Indian J. Plant Physiol. 2(2):142-144.
Lau, O. S. and Deng, X. W. 2010. Plant hormone signaling lightens up: integrators of light and hormones. Current Opinion in Plant Biol. 13(5):571-577. https://doi.org/10.1016/ j.pbi.2010.07.001. DOI: https://doi.org/10.1016/j.pbi.2010.07.001
Le B.; Do, N. T.; Jeanneau, M.; Sadik, S.; Tu, S.; Vidal, J. and Van, K. T. T. 1998. Rapid plant regeneration of a C4dicot species: Amaranthus edulis. Plant Sci. 132(1):45-54. https://doi.org/10.1016/S0168-9452(97)00262-8. DOI: https://doi.org/10.1016/S0168-9452(97)00262-8
Mnzava, N. A and Masam, A. M. 1985. Regeneration potential, leaf and seed yield of vegetable amaranth, (Amaranthus cruentus (L.), as a function of initial topping heights. Acta Hortic.9(153):151-160. https://doi.org/10.17660/ActaHortic.1985.153.20. DOI: https://doi.org/10.17660/ActaHortic.1985.153.20
Nasrin, F.; Jaber, P.; Alireza, M. A. and Saeideh, A. S. 2017. Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of persian shallot (Allium hirtifolium). Sci. Hortic. 218(2017):80-86. https://doi.org/10.1016/ j.scienta.2016.11.056. DOI: https://doi.org/10.1016/j.scienta.2016.11.056
Nhut, D. T.; Huy, N. P.; Tai, N. T.; Nam, N. B.; Luan, V. Q.; Hien, V. T.; Tung, H. T.; Vinh, B. T. and Luan, T. C. 2015. Light-emitting diodes and their potential in callus growth, plantlet development and saponin accumulation during somatic embryogenesis of Panax vietnamensis ha et grushv. Biotechnol. Biotechnol. Equip. 29(2):299-308. https://doi.org/10.1080/13102818.2014.1000210. DOI: https://doi.org/10.1080/13102818.2014.1000210
Osman, M. G.; Elhadi, E. A. and Khalafalla, M. M. 2010. Callus formation and organogenesis of tomato (Lycopersicon esculentum Mill, C. V. Omdurman) induced by thidiazuron. Afr. J. Biotechnol. 9(28):4407-4413. https://doi.org/10.5897/AJB10.1516. DOI: https://doi.org/10.5897/AJB10.1516
Ouyang, J.; Wang, X.; Zhao, B. and Wang, Y. 2003. Light intensity and spectral quality influencing the callus growth of Cistanche deserticola and biosynthesis of phenylethanoid glycosides. Plant Sci. 165(3):657-661. https://doi.org/10.1016/S0168-9452(03)00255-3. DOI: https://doi.org/10.1016/S0168-9452(03)00255-3
Pal, A.; Swain, S. S.; Das, A. B.; Mukherjee. A. K. and Chand P. K. 2013. Stable germ line transformation of a leafy vegetable crop amaranth (Amaranthus tricolor L.) mediated by Agrobacterium tumefaciens. In vitro Cell. Dev. Bio. Plant. 49(2):114-128. https://doi.org/ 10.1007/s11627-013-9489-9. DOI: https://doi.org/10.1007/s11627-013-9489-9
Rahman, M. A.; Alam, M. A.; Hossain, M. R.; Hossain, A. and Afroz, R. 2010. In vitro regeneration of popular tobacco varieties of Bangladesh from leaf disc. Bangladesh J. Agric. Res.35(1):125-134. https://doi.org/10.3329/bjar.v35i1.5873. DOI: https://doi.org/10.3329/bjar.v35i1.5873
Rajewski, A. C.; Elkins, K. B.; Henry, A.: Eck, J. V. and Litt, A. 2019. In vitro plant regeneration and Agrobacterium tumefaciens mediated transformation of Datura stramonium (Solanaceae). Appl. Plant Sci. 7(2):1-5. https://doi.org/10.1002/aps3.1220.
Scotton, D. C.; Benedito, V. A.; B de Molfetta, J. and Rodrigues, Benedita, I. F. P. 2013. Response of root explants to in vitro cultivation of marketable garlic cultivars. Hortic. Bras. 31(1):80-85. https://doi.org/10.1590/S0102-05362013000100013. DOI: https://doi.org/10.1590/S0102-05362013000100013
Shibasaki, N.; Hirose, K.; Yonemoto, T. and Tadaki, T. 1991. Suspension culture of Nicotiana tabacum cells in a rotary-drum bioreactor. J. Chem. Technol. Biotechnol. 53(4):359-363. https://doi.org/10.1002/jctb.280530407. DOI: https://doi.org/10.1002/jctb.280530407
Verma, S. K.; Das, A. K.; Cingoz, G. S.; Uslu, E. and Gurel, E. 2016. Influence of nutrient media on callus induction, somatic embryogenesis and plant regeneration in selected Turkish crocus species. Biotechnology Reports. 10(2016):66-74. https://doi.org/10.1016/j.btre. 2016.03.006. DOI: https://doi.org/10.1016/j.btre.2016.03.006
Wang, Y.; Zhang, H.; Zhao, B. and Yuan, X. 2001. Improved growth of Artemisia annua L hairy roots and artemisinin production under red light conditions. Biotechnology Letters. 23(2001):1971-1973. https://doi.org/10.1023/A:1013786332363. DOI: https://doi.org/10.1023/A:1013786332363
Wheeler, R. M. 2008. A historical background of plant lighting: an introduction to the workshop. HortSci. 43(7):1942-1943. https://doi.org/10.21273/HORTSCI.43.7.1942.
Yaacob, J. S.; Hwei, L. C. and Taha, R. M. 2012. Pigment analysis and tissue culture of Amaranthus cruentus L. Acta Hortic. 958(2012):171-178. https://doi.org/10.17660/ActaHortic.2012. 958.20. DOI: https://doi.org/10.17660/ActaHortic.2012.958.20
Yu, L.: Song, C.; Sun, L.; Li, L.; Xu, Z. and Tang, C. 2020. Effects of light-emitting diodes on tissue culture plantlets and seedlings of rice (Oryza sativa L.). J. Integrative Agric. 19(7):1743-1754. https://doi.org/10.1016/S2095-3119(19)62793-0.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


