Induction of androgenic embryos and regeneration of haploid plants in experimental genotypes of poblano chili through anthers culture
DOI:
https://doi.org/10.29312/remexca.v14i2.3054Keywords:
Capsicum annuum, homozygous, in vitroAbstract
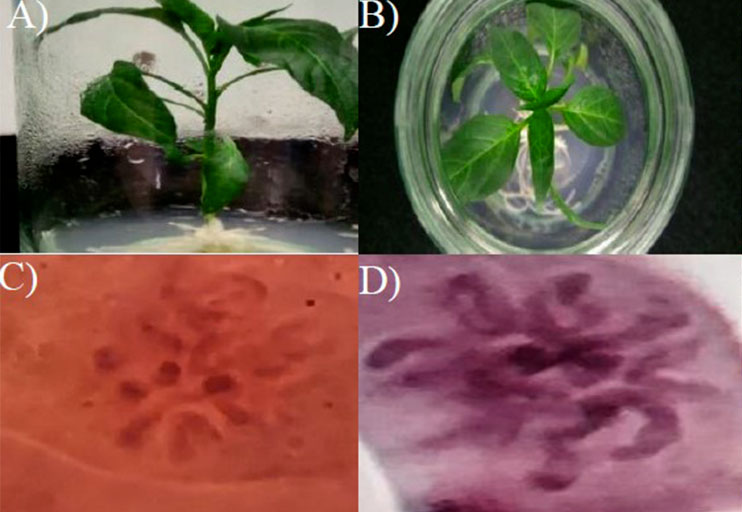
Traditional methods to obtain pure or homozygous lines involve several generations of self-fertilization with a significant time requirement. The obtaining of haploid and double haploid plants represents an alternative that reduces the production of lines with identical alleles in all their chromosomes to one generation. The objective of this work was to implement the protocol for obtaining androgenic embryos in populations of mulato and ancho chili of the chili improvement program in the Bajío Experimental Field of the National Institute of Forestry, Agricultural and Livestock Research during 2019 and 2020. Thirty chili genotypes included in the two populations mentioned below were subjected to androgen induction treatment. a) heterogeneous landrace population of mulato and ancho chilies with characteristic of cytoplasmic male sterility; and b) population of interspecific crosses of ancho chili with habanero chili (C. annuum x C. chinense) resistant to geminiviruses (PepGMV) and Phytophthora sp. The treatment consisted of N 6-furfuryladenine (kinetin) (0.01 mg L-1) and 2,4-dichlorophenoxyacetic acid (2,4-D) (0.01 mg L-1) for the induction of somatic embryogenesis and subsequently of kinetin (0.1 mg L-1) for induction of embryo germination. Chromosomal duplication was performed by colchicine (0.5%) prior to adaptation in vivo. The population made up of genotypes derived from interspecific crosses obtained greater efficiency of double haploid regenerated plants, between 4.29% and 14.67%, while the landrace population generated embryos in a smaller proportion, between 1.02% and 5.26%. These results are the first reported to obtain double haploid plants of mulato and ancho chilies.
Downloads
References
Ahloowalia, B. S. 1965. A root tip squash technique for screening chromosome-number in Lolium. Euphytica. 14(1):170-172. https://doi.org/10.1007/BF00038983.
Arjunappa, H. M.; Sateesh, K. P. and Prema, L. D. 2016. Studies on ploidy analysis and chromosome doubling in androgenic plants of chilli pepper (Capsicum annuum L.). Inter. J. Agric. Innov. Res. 4(4):627-633.
Ata, A.; Keles, D.; Taskin, H. and Buyukalaca, S. 2019. Effects of season, genotype, and nutrient medium on pepper anther culture and microspore development. Turk. J. Agric. For. 43(2):123-137. https://doi:10.3906/tar-1802-35.
Bajaj, Y. P. S. 1978. Regeneration of haploid tobacco plants from isolated pollen grown in drop culture. Indian J. Exp. Biol. 16(1):407-409.
Barany, I.; Gonzalez-Melendi, P.; Fadon, B.; Mityko, J.; Risueno, M. C. and Testillano, P. S. 2005. Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biol. Cell. 97(9):709-722.
Barany, I.; Testillano, P. S.; Mityko, J. and Risueno, M. C. 2001. The switch of the microspore developmental program in Capsicum involves HSP70 expression and leads to the production of haploid plants. Int. J. Dev. Biol. 45(S1):39-40.
Büyükalaca, S.; Comlekcioglu, N.; Abak, K.; Ekbic, E. and Kilic, N. 2004. Effects of silver nitrate and donor plant growing conditions on production of pepper (Capsicum annuum L.) haploid embryos via anther culture. Eur. J. Hort. Sci. 69(5):206-209.
Chambonnet, D. 1988. Production of haploid eggplant plants. Bulletin interne de la Station d’Amelioration des Plantes Maraicheres d’Avignon-Montfavet, France. 1-10 pp.
Comlekcioglu, N. 2021. Effect of colchicine addition to culture medium on induction of androgenesis in pepper (Capsicum annuum L.). Pak. J. Bot. 53(3):1001-1005. http://dx.doi.org/10.30848/PJB2021-3(14).
Comlekcioglu, N. and Ellialtıoğlu, S. S. 2018. Review on the research carried out on in vitro androgenesis of peppers (Capsicum annuum L.) in Turkey. Res. J. Biotech. 13(6):75-84.
Dumas de Vaulx, R.; Chambonnet, D. and Pochard, E. 1981. Culture in vitro d’anthères de piment (Capsicum annuum L.): amélioration des taux d’obtention de plantes chez différents génotypes par des traitements à + 35 °C. Agronomie, EDP Sciences. 1(10):859-864. https://hal.archives-ouvertes.fr/hal-00884205.
Ercan N and Ayar Şensoy, F. 2011. Androgenic responses of different Capsicum annuum L. cultivars, Biyoloji Bilimleri Arastirma Dergise. 4(2):59-61.
Gonzalez, M. P.; Testillano, P. S.; Prestamo, G.; Fadon, B. and Risueno, M. C. 1996. Cellular characterization of key developmental stage for pollen embryogenesis induction. Plant Dev. Biol. 127S-128S. http://hdl.handle.net/10261/252482.
Grozeva, S.; Rodeva, V.; Todorova, V. and Pundeva, R. 2009. Obtaining of pepper plants via anther culture. Genet. Breed. 38(1):25-31.
Jha, K.; Kumar, P. C. and Agarwal, A. 2021. Doubled haploid production in Capsicum annuum L. using anther culture: a review. Plant Archiv. 21(1):168-173. https://doi.org/10. 51470/plantarchives.2021.v21.S1.031.
Kim, M.; Kim, J.; Yoon, M.; Choi, D. and Lee, K. 2004. Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum). Plant Cell Tissue Organ Cult. 77(1):63-72.
Kristiansen, K. and Andersen, S. B. 1993. Effects of donor plant temperature, photoperiod, and age on another culture response of Capsicum annuum L. Euphytica. 67(1):105-109. https://doi.org/10.1007/BF00022732.
Munyon, I. P.; Hubstenberger, J. F. and Phillips, C. 1989. Origin of plantlets and callus obtained from chile pepper anther cultures. In vitro Cell. Dev. Biol. 25(3):293-296. https://doi.org/ 10.1007/BF02628469.
Niklas, N. A.; Olszewska, D.; Kisiała, A. and Nowaczyk, P. 2012. Study of individual plant responsiveness in anther cultures of selected pepper (Capsicum spp.) genotypes. Folia Hort. 24(2):141-146. https://doi:10.2478/v10245-012-0017-x.
Nowaczyk, L.; Nowaczyk, P. and Olszewska, D. 2016. Treating donor plants with 2,4-dicholophenoxyacetic acid can increase the effectiveness of induced androgenesis in Capsicum spp, Sci. Hortic. 205(23):1-6. https://doi.org/10.1016/j.scienta.2016.03.044.
Shimira, F.; Keleş, D.; Taşkın, H. and Abak, K. 2019. The assessment of androgenic response of two nematode resistant pepper (C. annuum L.) genotypes. Turkish J. Agric. Food Sci. Technol. 7(12):2103-2110. https://doi.org/10.24925/turjaf.v7i12.2103-2110.2828.
Snape, J. W. 1989. Doubled haploid breeding: theoretical basis and practical applications. In: review of advances in plant biotechnology 1985-1988. 2nd International Symposium on Genetic Manipulation in Crops. Mujeeb-Kazi A. and Sitch, L. A. Eds. (Mexico y Filipinas). International Maize and Wheat Improvement Center (CIMMYT) and International Rice Research Institute (IRRI). 19-30 pp.
Supena, E. D. J. and Custers, J. B. M. 2011. Refinement of shed-microspore culture protocol to increase normal embryos production in hot pepper (Capsicum annuum L.). Sci. Hor. 130(4):769-774. https://doi.org/10.1016/j.scienta.2011.08.037.
Testillano, P. S.; Coronado, M. J.; Segui, J. M.; Domenech, J.; González, M. P.; Raska, I. and Risueno, M. C. 2000. Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis. J Struct Biol. 129(2-3):223-232. https://doi.org/10.1006/ jsbi.2000.4249.
Touraev, A.; Vicente, O. and Heberle-Bors, E. 1997. Initiation of microspore embryogenesis by stress. Trends Plant Sci. 2(8):297-302. https://doi.org/10.1016/S1360-1385(97)89951-7.
Valladolid, A.; Blas, R. and Gonzáles, R. 2004. Introducción al recuento de cromosomas somáticos en raíces andinas. In: seminario J. Ed. Raíces Andinas. Contribuciones al conocimiento y la capacitación. Serie: conservación y uso de la biodiversidad de raíces y tubérculos andinos núm. 6. CIP. Agencia Suiza para el desarrollo y la cooperación. Lima, Perú. 96-99 pp.
Vivek, H.; Partap, P. S.; Yadav, R. C. and Baswana, K. S. 2017. In vitro androgenesis in Capsicum (Capsicum annuum L.). Int. J. Curr. Microbiol. App. Sci. 6(5):925-933. https://doi: https://doi.org/10.20546/ijcmas.2017.605.102.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


