Complejo PVA-quitosán-nCu mejora el rendimiento y la respuesta de defensa en tomate
DOI:
https://doi.org/10.29312/remexca.v12i6.3012Palabras clave:
bioestimulante, estrés, expresión de genes, hortalizasResumen
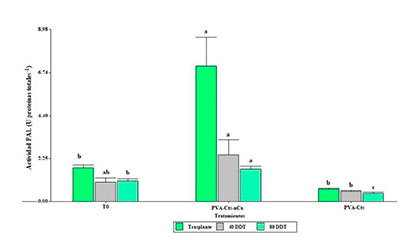
Actualmente el uso de la nanotecnología está revolucionando la producción agrícola. Se ha demostrado que las nanopartículas de cobre tienen un efecto en el crecimiento y desarrollo de las diferentes especies vegetales, además de operar como inductores de resistencia al estrés. El objetivo del presente trabajo fue evaluar la respuesta en el crecimiento y rendimiento, así como la activación del sistema de defensa de plantas de tomate. Los tratamientos evaluados fueron un complejo de alcohol polivinílico-quitosán-nanopartículas de cobre (PVA-Cts-nCu), otro complejo de PVA-Cts y un testigo absoluto (T0). Los tratamientos se aplicaron vía foliar en plantas de tomate bajo condiciones de invernadero. Durante el ciclo del cultivo, se determinaron variables agronómicas, y la actividad de enzimas relacionadas a la tolerancia a estrés como β-1,3 glucanasa, quitinasa y fenilalanina amonio liasa (PAL), así como la expresión del gen PR1. El complejo PVA-Cts-nCu incrementó el rendimiento, número de frutos, peso promedio de fruto, peso fresco aéreo y peso fresco de la raíz, además, promovió el sistema de defensa mediante el aumento en la actividad enzimática PAL, así como la sobreexpresión del gen PR1.
Descargas
Citas
AbuQamar, S.; Luo, H.; Laluk, K.; Mickelbart, M. V. and Mengiste, T. 2009. Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. The Plant J. 58(2):347-360. DOI: https://doi.org/10.1111/j.1365-313X.2008.03783.x
Adhikari, T.; Kundu, S.; Biswas, A. K.; Tarafdar, J. C. and Rao, A. S. 2012. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. 2(6A):815-823.
Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K. and Acharya, K. 2015. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Scientific reports. 5(1):1-14. DOI: https://doi.org/10.1038/srep15195
Chun, S. C. and Chandrasekaran, M. 2019. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Inter. J. Biol. Macromol. 125(51):948-954.
Cumplido-Nájera, C. F.; González-Morales, S.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2019. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. 245(1):82-89.
El Hadrami, A.; Adam, L. R.; El Hadrami, I. and Daayf, F. 2010. Chitosan in plant protection. Marine drugs. 8(4):968-987. DOI: https://doi.org/10.3390/md8040968
Falcón-Rodríguez, A.; Costales-Menéndez, D.; Martínez-Téllez, M. Á. y Gordon, T. A. 2012. Respuesta enzimática y de crecimiento en una variedad comercial de tabaco (Nicotiana tabacum, L.) tratada por aspersión foliar de un polímero de quitosana. Cultivos Tropicales. 33(1):65-70.
Fatima, F.; Hashim, A. and Anees, S. 2021. Efficacy of nanoparticles as nanofertilizer production: a review. Environ. Sci. Pollut. Res. 28(2):1292-1303.
Fish, W. W.; Perkins-Veazie, P. and Collins, J. K. 2002. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Composition and Analysis. 15(3):309-317. DOI: https://doi.org/10.1006/jfca.2002.1069
González-Peña, D.; Costales, D. y Falcón, A. B. 2014. Influencia de un polímero de quitosana en el crecimiento y la actividad de enzimas defensivas en tomate (Solanum lycopersicum L.). Cultivos Tropicales. 35(1):35-42.
Hernández, H H.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Hernández-Fuentes, A. D. and Juárez-Maldonado, A. 2017. Cu Nanoparticles in chitosan-PVA hydrogels as promoters of growth, productivity and fruit quality in tomato. Emirates J. Food Agric. 29(8):573-580.
Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G. and Juárez-Maldonado, A. 2018a. Effects of chitosan-PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules. 23(1):178-192. DOI: https://doi.org/10.3390/molecules23010178
Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D. and González-Morales, S. 2018b. Chitosan-PVA and copper nanoparticles improve growth and overexpress the SOD and JA genes in tomato plants under salt stress. Agronomy. 8(9):175-185.
Kasana, R. C.; Panwar, N. R.; Kaul, R. K. and Kumar, P. 2017. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Letters. 15(2):233-240. DOI: https://doi.org/10.1007/s10311-017-0615-5
López-Vargas, E. R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2018. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 8(7):1020-1034.
Padayatt, S. J.; Daruwala, R.; Wang, Y.; Eck, P. K.; Song, J.; Koh, W. S. and Levine, M. 2001. Vitamin C: from molecular actions to optimum intake. Handbook of antioxidants. CRC Press. Washington, DC. USA. 117-145. DOI: https://doi.org/10.1201/9780203904046.pt3
Pérez-Labrada, F.; López-Vargas, E. R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2019. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants. 8(6):151-167.
Pestovsky, Y. S. and Martínez-Antonio, A. 2017. The use of nanoparticles and nanoformulations in agriculture. J. Nanoscie. Nanotechnol. 17(12):8699-8730. DOI: https://doi.org/10.1166/jnn.2017.15041
Pinedo-Guerrero, Z. H.; Hernández-Fuentes, A. D.; Ortega-Ortiz, H.; Benavides-Mendoza, A. and Cadenas-Pliego, G. 2017. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules. 22(6):926. DOI: https://doi.org/10.3390/molecules22060926
Pradhan, S.; Patra, P.; Mitra, S.; Dey, K. K.; Basu, S.; Chandra, S.; and Goswami, A. 2015. Copper nanoparticle (CuNP) nanochain arrays with a reduced toxicity response: a biophysical and biochemical outlook on vigna radiata. J. Agric. Food Chem. 63(10):2606-2617. DOI: https://doi.org/10.1021/jf504614w
Rajput, V. D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V. and Fedorenko, A. 2018. Effects of copper nanoparticles (CuO NPs) on crop plants: a mini review. Bionanoscience. 8(1):36-42. DOI: https://doi.org/10.1007/s12668-017-0466-3
Ramos, S. J.; Faquin, V.; Guilherme, L. R. G.; Castro, E. M.; Ávila, F. W.; Carvalho, G. S. and Oliveira, C. 2010. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 56(12):584-588. DOI: https://doi.org/10.17221/113/2010-PSE
Rodríguez, A. T.; Ramírez, M. A.; Falcón, A.; Utria, E. y Bautista, S. 2006. Estimulacion de algunas enzimas en plantas de arroz (Oryza sativa, L.) tratadas con un hidrolizado de quitosana. Cultivos Tropicales. 27(2):87-91.
Rodríguez-Pedroso, A. T.; Ramírez-Arrebato, M. Á.; Cárdenas-Travieso, R. M.; Falcón-Rodríguez, A. y Bautista-Baños, S. 2006. Efecto de la quitosana en la inducción de la actividad de enzimas relacionadas con la defensa y protección de plántulas de arroz (Oryza sativa L.) contra Pyricularia grisea Sacc. Rev. Mex. Fitopatol. 24(1):1-7.
Rodríguez-Pedroso, A. T.; Ramírez-Arrebato, M. A.; Rivero-González, D.; Bosquez-Molina, E.; Barrera-Necha, L. L. y Bautista-Baños, S. 2009. Propiedades químico-estructurales y actividad biológica de la quitosana en microorganismos fitopatógenos. Rev. Chapingo. Ser. Hortic. 15(3):307-317.
Rouphael, Y. and Colla, G. 2020. Biostimulants in agriculture. Frontiers in Plant Sci. 11(1):40-47.
Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M. K.; Sharma, S. S.; Pal, A. and Biswas, P. 2015. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Inter. J. Biol. Macromol. 75(1):346-353. DOI: https://doi.org/10.1016/j.ijbiomac.2015.01.027
Sánchez, G. R.; Mercado, E. C.; Peña, E. B.; Cruz, H. R. y Pineda, E. G. 2010. El ácido salicílico y su participación en la resistencia a patógenos en plantas. Biológicas. 12(2):90-95.
Santos-Sánchez, N. F.; Salas-Coronado, R.; Villanueva-Cañongo, C. and Hernández-Carlos, B. 2019. Antioxidant compounds and their antioxidant mechanism. London, UK. IntechOpen. 1-28 p.
Sierra-Ávila, R. M. P.; Cadenas-Pliego, G.; Avila-Orta, C.; Betancourt, R.; Jiménez-Regalado, E.; Jiménez-Barrera, R. and Martínez, G. 2014. Synthesis of copper nanoparticles coated with nitrogen ligands. J. Nanomater. 2014(1):74-82. DOI: https://doi.org/10.1155/2014/361791
Sierra-Ávila, R.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Comparán Padilla, V.; Ávila-Orta, C.; Pérez Camacho, O.; Jiménez-Regalado, E.; Hernández-Hernández, E. and Jiménez-Barrera, R. M. 2015. Synthesis of copper nanoparticles using mixture of allylamine and polyallylamine. J. Nanomaterials. 361797(1):1-8. DOI: https://doi.org/10.1155/2015/367341
Somasundaran, P.; Fang, X.; Ponnurangam, S. and Li, B. 2010. Nanoparticles: Characteristics, mechanisms and modulation of biotoxicity. KONA powder and particle journal. 28(1):38-49. DOI: https://doi.org/10.14356/kona.2010007
Steiner, A. A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 15(2):134-154. DOI: https://doi.org/10.1007/BF01347224
Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M. R.; Kawiak, A.; Jeziorek, M.; Wyderska, S. and Chinou, I. 2012. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In vitro Cellular & Developmental Biology-Plant. 48(5):555-564. DOI: https://doi.org/10.1007/s11627-012-9443-2
Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S. A.; ur Rehman, H. and Sanaullah, M. 2020. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 721(1):137778.
Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J. S.; Bruni, G.; Kozanecki, M. and Puglia, D. 2018. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydrate Polymers. 181(1):275-284. DOI: https://doi.org/10.1016/j.carbpol.2017.10.084
Yang, W.; Owczarek, J. S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G. M. adn Puglia, D. 2016. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Industrial Crops and Products. 94(1):800-811. DOI: https://doi.org/10.1016/j.indcrop.2016.09.061
Yu, Z. and Dahlgren, R. A. 2000. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 26(9):2119-2140. DOI: https://doi.org/10.1023/A:1005568416040
Zhang, X. and Liu, C. J. 2015. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant. 8(1):17-27. DOI: https://doi.org/10.1016/j.molp.2014.11.001
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2021 Revista Mexicana de Ciencias Agrícolas

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores(as) que publiquen en Revista Mexicana de Ciencias Agrícolas aceptan las siguientes condiciones:
De acuerdo con la legislación de derechos de autor, Revista Mexicana de Ciencias Agrícolas reconoce y respeta el derecho moral de los autores(as), así como la titularidad del derecho patrimonial, el cual será cedido a la revista para su difusión en acceso abierto.
Los autores(as) deben de pagar una cuota por recepción de artículos antes de pasar por dictamen editorial. En caso de que la colaboración sea aceptada, el autor debe de parar la traducción de su texto al inglés.
Todos los textos publicados por Revista Mexicana de Ciencias Agrícolas -sin excepción- se distribuyen amparados bajo la licencia Creative Commons 4.0 atribución-no comercial (CC BY-NC 4.0 internacional), que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista.
Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en Revista Mexicana de Ciencias Agrícolas (por ejemplo incluirlo en un repositorio institucional o darlo a conocer en otros medios en papel o electrónicos) siempre que indique clara y explícitamente que el trabajo se publicó por primera vez en Revista Mexicana de Ciencias Agrícolas.
Para todo lo anterior, los autores(as) deben remitir el formato de carta-cesión de la propiedad de los derechos de la primera publicación debidamente requisitado y firmado por los autores(as). Este formato debe ser remitido en archivo PDF al correo: revista_atm@yahoo.com.mx; revistaagricola@inifap.gob.mx.
Esta obra está bajo una licencia de Creative Commons Reconocimiento-No Comercial 4.0 Internacional.


