Stability of the explant in the in vitro proliferation of axillary shoots of the biznaga
DOI:
https://doi.org/10.29312/remexca.v13i1.2309Keywords:
in vitro culture, growth regulators, ornamental cactiAbstract
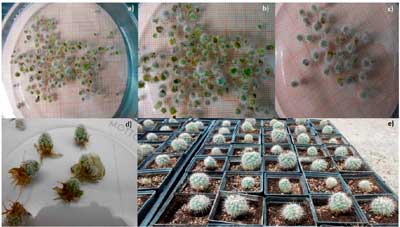
To conserve ex situ, promote the use and enhancement of native germplasm of ornamental interest such as T. viereckii subsp. major, a species at risk Pr, the present research was proposed with the aim of regenerating this species in the Plant Tissue Culture Laboratory (LCTV) of the Saltillo CIRNE-INIFAP Experimental Field considering the stability of the explant during subsequent subcultures, determine the type and concentration of phytohormones and the best cytokinin-auxin ratio that most influence the in vitro multiplication stage. The intermediate segments of the stem (SIT) obtained from vitroplants was used as an explant. From 2017 to 2019, the multiplication stage was evaluated using the Murashige-Skoog medium (MS; Murashige and Skoog, 1962) at 50% of its macrosalts. A completely randomized experimental design with factorial arrangement was used to evaluate two cytokinins: 6-benzyl aminopurine (BAP) and 6-furfuryl aminopurine (KIN) at four concentrations 2.5, 5, 7.5 and 10 mg L-1 in interaction with two auxins: naphthaleneacetic acid (NAA) and indole butyric acid (IBA) in a cytokinin-auxin ratio of 10:1. In total, 16 treatments were evaluated, establishing five SIT/bottle with 10 repetitions/treatment. This evaluation was repeated five times by means of subcultures, evaluating the number of shoots/explant (Nb) and the shoot height (Ab, mm) every ten weeks. The analysis of variance (Anova) showed that there was stability of the explant during the subcultures, maintaining a similar multiplication rate for more than three years, an important effect for intensive multiplication. When analyzing the treatments as independent effects in the Tukey mean test (p≤ 0.05), the interactions BAP: IBA and KIN: IBA with concentrations of 5 mg L-1 BAP + 0.50 mg L-1 IBA and 2.5 mg L-1 KIN + 0.25 mg L-1 IBA were selected as the treatments that induce shoots with a multiplication rate of 9.25 shoots/explant with an Ab of 6.73 mm.
Downloads
References
Aggarwal, D.; Upadhyay, S. K.; Kumar, K.; Sehrawat, N.; Tuli, H. S. and Singh, R. 2018. Effects of plant growth regulators on in vitro propagation of economically important ornamental plant Rosa hybrida L. Asian J. Biol Life Sci. 9(2):227-233. Doi :10.5530/ajbls.2018.9.35.
Ascough, G. D. and Staden, V. J. 2010. Micropropagation of Albuca bracteata and A. nelsonii indigenous ornamentals with medicinal value. South Afr. J. Bot. 76(3):579-584. https://www.researchgate.net/publication/337387811.
Bianchetti, R. E.; Ferrara, R. C.; Sacramento, P. V.; Floriano, D. F.; Mystica, S. de O A.; Condé, E. F J. and Pereira, P. P. H. 2017. An improved protocol for in vitro propagation of the medicinal plant Mimosa pudica L. Afr. J. Biotechnol. 16(9):418-428. Doi:10.5897/ AJB2016.15831.
Cantos-Cevallos, G.; Pinargote-Choez, J. y Palma-Ponce R. 2018. Influencia de la fitohormona kinetina en el crecimiento de plántulas de Coffea arabiga L. injertadas sobre patrón robusta en vivero. Rev. Cubana Ciencias Forestales. Pinar del Río. 6(2):134-145. http://scielo.sld. cu/scielo.php?script=sci-arttext&pid=s231034692018000200134&lng=es&nrm=iso.
Cardarelli, M.; Borgognone, D. y Colla, G. 2010. Propagación in vitro de Obregonia denegrii (Cactaceae). Propagación de plantas ornamentales. 10(1):29-36. https://www.researchgate. net/publication/262261883.
Castro-Gallo, I.; Meza-Rangel, E.; Pérez-Reyes, M. E. y Pérez-Molphe, B. M. E. 2002. Propagación in vitro de 10 especies mexicanas de cactáceas. Sciantiae Naturae. 4(2):5-24.
Choreño, T. J.; González, M. R. H.; Terrazas, T. S. y Hernández, L. A. 2002. Propagación in vitro de Cephalocereus senilis Haworth Pfeiffer a partir de areolas. Rev. Chapingo. Ser. Hortic. 8(2):183-196. https://doi.org/10.5154/r.rchsh.2001.01.008.
Dávila, F. C. A.; De La Rosa-Carrillo, M. L. and Pérez, B. M. 2005. In vitro propagation of eight species or subspecies of Turbinicarpus (Cactaceae). In vitro Cel. Dev. Biology-Plant. 41(4):540-545. https://doi.org/10.1079/IVP2005668.
De la Rosa-Carrillo, M. L.; Domínguez-Rosales, M. S.; Pérez-Reyes, M. E. y Pérez-Molphe-Balch, E. 2012. Cultivo y propagación in vitro de cactáceas amenazadas del género Turbinicarpus. Interciencia. 37(2):114-120. http://www.redalyc.org/articulo.oa?id=33922717006.
Espinoza-Flores, A.; González-Rosas, H. y Mejía-Muñoz, J. 2003. La comercialización de plantas en peligro de extinción. In: plantas nativas de México con potencial ornamental. Universidad Autónoma, Chapingo (UACH). AMEHOAC. Asociación Mexicana de Horticultura Ornamental, AC. Texcoco, Estado de México, México. 199-217 pp.
Finti, I. A.; Boullani, E. R.; Ayadi, E. F.; Aabd, A. N. and Mousadik, E. A. 2012. Micropropagation in vitro of Opuntia Ficus-indica in south of Morocco. Int. J. Chem. Biochem. Sci. 1:6-10. http://www.iscientific.org/wp-content/uploads/2018/02/2-IJCBS-12-1-06.pdf.
Gámez, M.; Villavicencio, O. E. E. G.; Serrato, M. A. C.; Mejía, J. M. M.; Treviño, G. C.; Martínez, L. G.; Rodríguez, M. O.; Granada, L. C.; Flores, M. C.; Reyes, J. S.; Islas, M. L.; Salome, E. C.; Menchaca, R. A. G.; Espadas, C. M. M.; Hernández, L. S.; Vázquez, L.M. C.; Martínez, G. F. M.; Vargas, O. P. y Ríos, E. S. 2016. Conservación y aprovechamiento sostenible de especies ornamentales nativas de México. Servicio Nacional de Inspección y Certificación de Semillas (SNICS)-Universidad Autónoma Chapingo (UACH). Texcoco, Estado de México, México. 152 p. https://www.gob.mx/cms/uploads/attachment/ file/172778/Conservaci-n-y-aprovechamiento-sostenible-de-especies-ornamentales-de-M-xico.pdf.
Giusti, P.; Vitti, D.; Fiocchetti, F.; Colla, G.; Saccardo, F. and Tucci, M. 2002. In vitro propagation of three endangered cactus species. Sci. Hortic. 95(4):319-332. https://www.sciencedirect.com/science/article/abs/pii/S0304423802000316?via%3Dihub.
Gonçalves, M. L.; Machado, P. S. M. de Fátima.; Ballesta, P.; Mora, F.; Milaneze, G. M. A. y Aparecida, M. C. 2016. Suplementos orgánicos para el cultivo in vitro del híbrido Laeliocattleya (Orchidaceae). Idesia. 1(34):47-54. http://dx.doi.org/10.4067/S0718-34292016000100006.
Granada, C. L. 2014. La importancia del sector ornamental como un potencial de alto contenido de participación social. In: primer simposio nacional plantas ornamentales nativas mexicanas con potencial comercial. SNICS-SINAREFI. Tezoyuca. Morelos México. 10 p.
Guzmán, U.; Arias, M. S. y Dávila, P. P. 2003. Catálogo de cactáceas mexicanas. Universidad Nacional Autónoma de México (UNAM)- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). México, DF. México. 315 p. https://www.worldcat.org/ title/catalogo-de-cactaceas-mexicanas/oclc/948238461.
Hunt, D. 2006. The new cactus lexicon. descriptions & illustrations of the cactus family. Compiles and edited by members or the International Cactaceae Systematic Group. England. (Ed.). 373 p. https://www.nhbs.com/the-new-cactus-lexicon-volumes-i-and-ii-book.
Martínez-Villegas, Y. M.; Andrade-Rodríguez, M.; Villegas-Monter, A.; Alia-Tejacal, I.; Villegas-Torres O. G. y López-Martínez, V. 2011. Cultivo in vitro de pitayo (Stenocereus stellatus [Pfeiffer] Riccobono). Rev. Chapingo Hortic. 17(3):95-105. https://doi.org/10.5154/r. rchsh. 2011.17.021.
Mata-Rosas, M.; Monroy-De la Rosa, M. A.; Moebius-Goldammer, K. and Chávez-Ávila, V. M. 2001. Micropropagation of Turbinicarpus laui Glass et Foster, an endemic and endangered species. In vitro Cell. Dev. Pl. 37:400-404. https://doi.org/10.1007/s11627-001-0070-6.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiologia Plantarum. 15(3):473-477. http://dx.doi.org/10.1111/j.1399-3054.1962.tb08052.x.
Ordoñez, M. M. A. 2003. Propagación in vitro de Mammillaria voburnensis Scheer. (Cactaceae) Universidad de San Carlos de Guatemala. Facultad de Ciencias Químicas y Farmacia. Guatemala. 70 p. http://biblioteca.usac.edu.gt/tesis/06/06-2162.pdf.
Pérez-Molphe-Balch, E.; Pérez-Reyes, M. E.; Villalobos-Amador, E.; Meza-Rangel, E.; Morones-Ruiz, L. R. and Lizalde-Viramontes, H. J. 1998. Propagation of 21 species of mexican cacti by axillary proliferation. In vitro Cell. Dev. Pl. 34(2):131-135. https://doi.org/10.1007/BF02822777.
Pérez-Molphe-Balch E. and Dávila-Figueroa, C. A. 2002. In vitro propagación de Pelecyphora aselliformis Ehrenberg y P. strobiliformis Werdermann (Cactaceae). In Vitro Cel. Develop. Biol. Plant. 38(1):73-78. https://link.springer.com/article/10.1079%2FIVP2001248.
Pérez-Molphe-Balch, E.; Santos-Díaz, M. S.; Ramírez-Malagón, R. y Ochoa-Alejo, N. 2015. Cultivo de tejidos de cactus ornamentales. Sci. Agric. 72(6):540-561. https://www.scielo.br/j/sa/a/VtcbrDrxhm8L3yLFDcfV46j/?lang=en.
Ramírez-Malagón, R.; Aguilar-Ramírez, I.; Borodanenko, A.; Pérez-Moreno, L.; Barrera-Guerra, J. L.; Nulez-Palenius, H. G. and Ochoa-Alejo, N. 2007. In vitro propagation of ten thratened species of Mammillaria (Cactaceae). In vitro Cell. Dev. Pl. 43(6):660-665. https://agris.fao.org/agris-search/search.do?recordID=US201500203765.
SEMARNAT. 2010. Secretaría del Medio Ambiente y Recursos Naturales. Norma Oficial Mexicana NOM-059-ECOL-2010. Protección ambiental-especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio. Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010. México, DF. 130 p. https://dof.gob.mx.
Soltero, Q. R. y Portillo L. 2015. Micropropagación de cactáceas mexicanas amenazadas. Bol. Nakari. 26(2):13-17. https://www.researchgate.net/publication/285204536.
Soria-Campos, D.; López-Escamilla, A. L. y Olguín-Santos, L. P. 2013. Propagación in vitro de Mammillaria schiedeana schiedeana (Cactaceae), subespecie endémica y amenazada de extinción de la Barranca de Metztitlán, Hidalgo. Estudios científicos en el estado de Hidalgo y zonas aledañas. Lincoln, NE. Zea Books. 2(16):121-128. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1015&context=hidalgo.
SAS. 2019. Statistical Analysis System Institute Inc. SAS/STAT®14.1 User’s guide second edition. SAS Institute Inc. Raleigh, NC, USA. 238 p.
Tropicos. 2019. Trópicos: Turbinicarpus viereckii subsp. major (Glass & R.A. Foster) Glass). http://www.tropicos.org/Name/33700793.
Velázquez E. L. E. y Soltero, Q. R. 2001. Micropropagación de Ephithelantha micromeris Engelm. Weber ex Britton et Rose. 46(3):56-62.
Villanueva, F.; Ávila, M.; Mansilla, A.; Abades, S. y Cáceres, J. 2013. Efecto de auxinas y citoquininas en el cultivo de tejido de Ahnfeltia plicata (Hudson) Fries, 1836 (Ahnfeltiales, rhodophyta) de la región de Magallanes. Anales Instituto Patagonia. Punta Arenas, Chile. 1(41):99-111. http://dx.doi.org/10.4067/S0718-686X2013000100009.
Villavicencio-Gutiérrez, E. E.; González-Cortes, A.; Arredondo-Gómez, A.; Iracheta-Donjuan, L.; Comparan-Sánchez, S. y Casique-Valdés, R. 2011. Micropropagación de Turbinicarpus knuthianus (Boed.) John & Riha cactácea ornamental, amenazada de extinción del Desierto Chihuahuense. Rev. Mex. Cien. Forest. 6(2):37-56. https://doi.org/10.29298/rmcf. v2i6.573.
Villavicencio, G. E. E.; González, C. A. y Carranza, P. M. A. 2012. Micropropagación de Epithelantha micromeris (Engelm.) F.A.C. Weber ex Britt. & Rose cactácea ornamental y recurso fitogenético del Desierto Chihuahuense. Rev. Mex. Cien. Forest. 14(3):83-99. Doi: https://doi.org/10.29298/rmcf.v3i14.476.
Villavicencio, G. E. E.; Arellano, O. G. y Belmontes, D. V. A. 2020. Micropropagación del órgano pequeño sacasil (Echinocereus poselgeri Lem.) (Fam.: Cactaceae). Acta Hortic. 1288(1):123-130. Doi: 10.17660/ActaHortic.2020.1288.19.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


