Evaluar el efecto del extracto de chipilín y alcaloide pirrolizidinico de dietas líquidas en Bactericera cockerelli
DOI:
https://doi.org/10.29312/remexca.v16i8.3920Palabras clave:
Crotalaria longirostrata, alcaloide pirrolizidínico, bioensayos de alimentación, psílido del tomateResumen
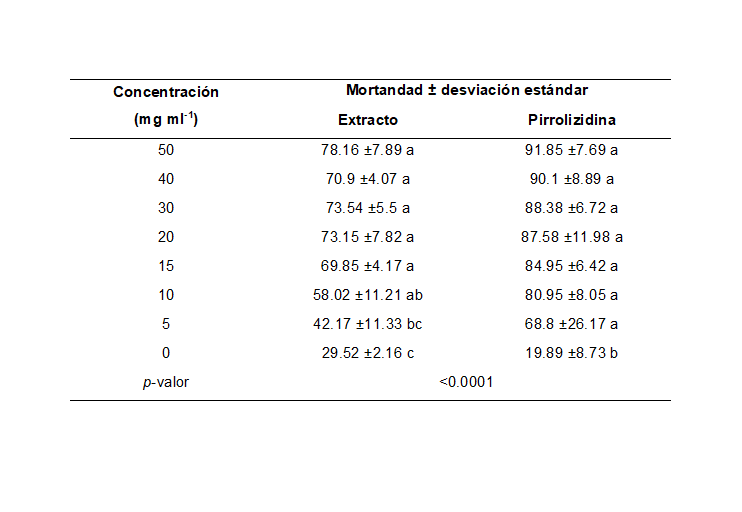
Las plagas Bactericera cockerelli es una de mayor importancia económica y más destructivas en cultivos de la familia de las solanáceas, donde el control químico ha sido la principal estrategia de manejo. El uso de extractos botánicos es una alternativa bioracional al manejo de esta plaga. La actividad insecticida del género Crotalaria se atribuye a la presencia de alcaloides, saponinas, flavonoides y alcaloides pirrolizidínicos. El objetivo de este trabajo fue evaluar el efecto insecticida del extracto de C. longirostrata y la fracción del alcaloide pirrolizidínico (1β,2β-Epoxy-1α-methoxymethyl-8α-pyrrolizidina), mediante el suministro de dietas líquidas en B. corekerelli. Los bioensayos de alimentación en dietas líquidas se desarrollaron en el Laboratorio de Toxicología de la Universidad Autónoma Agraria Antonio Narro. En cámaras de alimentación de plástico se implementó una dieta líquida suplementada con 5, 10, 15, 20, 30, 40 y 50 mg ml-1 del extracto metanólico de C. longirostrata y el fraccionado del alcaloide pirrolizidínico, donde se evaluó la mortalidad bajo un diseño completamente al azar. Se determinó la CL50 para la fracción 1β,2β-Epoxy-1α-methoxymethyl-8α-pyrrolizidina y el extracto metanólico de chipilín, obteniendo una mortalidad en el extracto metanólico entre el 42-78%, mientras que la fracción del alcaloide pirrolizidínico registró una mortalidad del 68-91%; siendo este último el que presentó una CL50 menor. El extracto metanólico de chipilín y la fracción del alcaloide pirrolizidínico presentaron actividad insecticida en las dietas líquidas, demostrando eficiencia para su uso en el control de B. cockerelli.
Descargas
Citas
Barrios-Díaz, B.; Arellano-Fuentes, M. E.; Vázquez-Huerta, G.; Barrios-Díaz, J. M.; Berdeja-Arbeu, R. y Hernández-Tapia, M. R. 2016. Control alternativo de paratrioza (Bactericera cockerelli Sulc.) en chile serrano (Capsicum annuum L.). Entomología Mexicana. 3(1):146-152. https://www.acaentmex.org/entomologia/revista/2016/AGR/Em%20146-152.pdf.
Cadena, H. M. A. 1993. La punta morada de la papa en México: Incidencia y búsqueda de resistencia. Agrociencia. 4(1):247-256.
Cerna, C. E.; Ail, C.; Landeros, J.; Sánchez, S.; Badii, M.; Aguirre, L. and Ochoa, Y. 2012. Comparison of toxicity and selectivity of the pest Bactericera cockerelli and its predator Chrysoperla carnea. Agrociencia. 46(8):783-793. https://www.scielo.org.mx/pdf/agro/v46n8/v46n8a4.pdf.
Cerna, C. E.; Beltrán, B. M.; Ochoa, Y. M.; Hernández, B. O. and Delgado, O. J. C. 2021. Bactericera cockerelli vector de Candidatus Liberibacter solanacearum, morfometría y haplotipos en poblaciones de México. Revista Mexicana de Ciencias Agrícolas. 26(1):81-94. https://doi.org/10.29312/remexca.v0i26.2939.
Cerna, C. E.; Hernández, O.; Landeros, J.; Aguirre, L. A. and Ochoa, Y. M. 2015. Insecticide-resistance ratios of three populations of Bactericera cockerelli (Hemiptera: Psylloidea: triozidae) in regions of northern Mexico. Florida Entomologist. 98(3):950-953. https://doi.org/10.1653/024.098.032.
Fürstenberg-Hägg, J.; Zagrobelny, M. and Bak, S. 2013. Plant defense against insect herbivores. International Journal of Molecular Sciences. 14(5):10242-10297. https://doi.org/10.3390/ijms140510242.
Greenway, G. A. and Rondon, S. 2018. Economic impacts of zebra chips in Idaho, Oregon, and Washington. American Journal of Potato Research. 95(1):362-367. https://doi.org/10.1007/s12230-018-9636-2.
Gudmestad, N. C. and Secor, G. A. 2007. Zebra chip: a new disease of potato. Nebraska Potato Eyes. 19(1):1-4. https://www.ndsu.edu/fileadmin/potatopathology/potato-trials/Zebra-Chip-New-Potato-Disease.pdf.
Gutiérrez-Ramírez, J. A.; Betancourt-Galindo, R.; Aguirre-Uribe, L. A.; Cerna-Chávez, E.; Sandoval-Rangel, A.; Castro-del Ángel, E.; Chacón-Hernández, J. C.; García-López, J. I. and Hernández-Juárez, A. 2021. Insecticidal effect of zinc oxide and titanium dioxide nanoparticles against Bactericera cockerelli Sulc. (Hemiptera: Triozidae) on Tomato Solanum lycopersicum. Agronomy. 11(8):1460-1469. https://doi.org/10.3390/agronomy11081460.
Henderson, C. F. and Tilton, E. 1955. Tests with acaricides against the brown wheat mite. Journal of Economic Entomology. 48(2):157-161. https://doi.org/10.1093/jee/48.2.157.
Hernández, O. L.; Carranza, R. P.; Cobos, P. L. E.; López, L. L. I.; Ascasio, V. J. A. and Silva, B. S. Y. 2017. Bioguided fractionation from Solanum elaeagnifolium to evaluate toxicity on cellular lines and breast tumor explants. Vitae. 24(2):124-131. https://doi.org/10.17533/udea.vitae.v24n2a05.
Hernández-Reyes, A. J. Guzmán-Albores, M.; León-Rodríguez, A.; Ruíz-Valdiviezo, V. M.; Rodríguez-Ortiz, L. R. and Barba-Rosa, A. P. 2024. Toxicological and sedative effects of chipilin (Crotalaria longirostrata) leaf extracts obtained by maceration and supercritical fluid extraction. ACS Omega. 9(17):18862-18871. https://doi.org/10.1021/acsomega.3c08290.
Kolomiiets, Y. V.; Grygoryuk, I. P.; Butsenko, L. M. and Kalinichenko, A. V. 2019. Biotechnological methods control phytopathogenic bacteria in Tomatoes. Applied Ecology and Environmental Research. 17(2):3215-3230. http://dx.doi.org/10.15666/aeer/1702-32153230.
López López. H.; Beltrán, B. M.; Ochoa, Y. M.; Castro, E.; Cerna, E. y Delgado, J. C. 2022. Extracto metanólico de Crotalaria longirostrata: identificación de metabolitos secundarios y su efecto insecticida. Scientia Agropecuaria. 13(1):71-78. http://dx.doi.org/10.17268/sci.agropecu.2022.007.
Miranda-Granados, J.; Chacón, C.; Ruiz-Lau, N.; Vargas-Díaz, M. E.; Zepeda, L. G.; Alvarez-Gutiérrez, P.; Meza-Gordillo, R. and Lagunas-Rivera, S. 2018. Alternative use of extracts of chipilín leaves (Crotalaria longirostrata Hook. & Arn) as antimicrobial. Sustainability. 10(3):883-891. https://doi.org/10.3390/su10030883.
Morton, J. F. 1994. Pito (Erythrina berteroana) and chipilin (Crotalaria longirostrata), (fabaceae) two soporific vegetables of Central America. Economic Botany. 48(2):130-138. https://doi.org/10.1007/BF02908199.
Mutale-joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y. and Hicham, E. 2020. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Scientific Reports. 10:2820-821. https://doi.org/10.1038/s41598-020-59840-4.
Obembe, O. M. and Kayode, J. 2013. Insecticidal activity of the aqueous extracts of four under-utilized tropical plants as protectant of cowpea seeds from Callosobruchus maculatus infestation. Pakistan Journal of Biological Sciences. 16(4):175-179. https://doi.org/10.3923/pjbs.2013.175.179.
Oh, J. and Tamborindeguy, C. 2023. Treatment of rapamycin and evaluation of an autophagic response in the gut of Bactericera cockerelli (Sulč). Insects. 14(2):142-151. https://doi.org/10.3390/insects14020142.
Olaniyan, O.; Rodríguez-Gasol, N.; Cayla, N.; Michaud, E. and Wratten, S. D. 2020. Bactericera cockerelli (Sulc), a potential threat to China’s potato industry. Journal of Integrative Agriculture. 19(7):338-349. https://doi.org/10.1016/S2095-3119(19)62754-1.
Peñaloza, A. G. C. y Peláez, J. C. A. 2014. Evaluación de la actividad biológica de extractos de semillas de Crotalaria pallida (cascabelito) sobre el modelo Drosophila melanogaster. Revista Cubana de Plantas Medicinales. 19(3):144-153.
Prager, S. M. and Trumble, J. T. 2018. Psyllids: biology, ecology and management. In Sustain. Manag. Arthropod Pests Tomato. 163-181. https://doi.org/10.1016/B978-0-12-802441- 6.00007-3.
Ramesh, N. G. 2020. Iminosugars. In: carbohydrates in drug discovery and development. Tiwari, V. K. Ed. 331-381 pp. Elsevier Inc. https://doi.org/10.1016/B978-0-12-816675-8.00008-7.
Rech, C.; Ribeiro, L. P.; Bento, J. M. S.; Pott, C. A. and Nardi, C. 2022. Monocrotaline presence in the Crotalaria (Fabaceae) plant genus and its influence on arthropods in agroecosystems. Brazilian Journal of Biology. 84:256916. https://doi.org/10.1590/1519-6984.256916.
Rivera-Martínez, R.; Ramírez-Dávila, J. F. y Acosta-Guadarrama, A. D. 2018. Distribución espacial de las poblaciones de huevos de Bactericera cockerelli Sulc. en el cultivo de tomate de cáscara (Physalis ixocarpa Brot.). Acta Universitaria. 28(5):24-33. https://doi.org/10.15174/au.2018.1944.
Roque-Enríquez, A.; Delgado-Ortiz, J. C.; Beltrán-Beache, M.; Ochoa-Fuentes, Y. y Cerna-Chávez, E. 2021. Parámetros agronómicos del tomate (Solanum lycopersicum L.) inoculado con “Candidatus Liberibacter solanacearum” y tratados con fosfitos. Ecosistemas y Recursos Agropecuarios. 8(1):2552. https://doi.org/10.19136/era.a8n1.2552.
Servicio de Información Agroalimentaria y Pesquera. 2024. Anuario Estadístico de la Producción Agrícola. https://nube.siap.gob.mx/cierreagricola/.
Shukla, E.; Thorat, L. J.; Nath, B. B. and Gaikwad, S. M. 2015. Insect trehalase: physiological significance and potential applications. Glycobiology. 25(4):357-367. https://doi.org/10.1093/glycob/cwu125.
Sumner, K. J. C.; Highet, F.; Arnsdorf, Y. M.; Back, E.; Carnegie, M.; Madden, S.; Carboni, S.; Billaud, W.; Lawrence, Z. and Kenyon, D. 2020. ‘Candidatus Liberibacter solanacearum’ distribution and diversity in Scotland and the characterization of novel haplotypes from Craspedolepta spp. (Psylloidea: aphalaridae). Scientific Reports. 10(9):16567. https://doi.org/10.1038/s41598-020-73382-9.
Swisher, K. D.; Arp, A. P.; Bextine, B. R.; Álvarez, E. A.; Crosslin, J. M. and Munyaneza, J. E. 2013. Haplotyping the potato psyllid, Bactericera cockerelli, in Mexico and Central America. Southwestern Entomologist. 38(2):201-208. https://doi.org/10.3958/059.038.0205.
Swisher, K. D.; Munyaneza, J. E.; Velásquez-Valle, R. and Mena-Covarrubias, J. 2018. Detection of pathogens associated with psyllids and leafhoppers in Capsicum annuum L. in the Mexican states of Durango, Zacatecas and Michoacan. Plant Disease. 102(1):146-153. https://doi.org/10.1094/PDIS-05-17-0758-RE.
Tamburino, R.; Sannino, L.; Cafasso, D.; Cantarella, C.; Orrù, L.; Cardi, T.; Cozzolino, S.; D’Agostino, N. and Scotti, N. 2020. Cultivated tomato (Solanum lycopersicum L.) suffered a severe cytoplasmic bottleneck during domestication: Implications from chloroplast genomes. Plants. 9(10):1443. https://doi.org/10.3390/plants9111443.
Tang, X. T.; Longnecker, M. and Tamborindeguy, C. 2020. Acquisition and transmission of two ‘Candidatus Liberibacter solanacearum’ haplotypes by the tomato psyllid Bactericera cockerelli. Scientific Reports. 10(13):14000. https://doi.org/10.1038/s41598-020-70795-4.
Thoden, T. C.; Boppré, M. and Hallmann, J. 2009. Effects of pyrrolizidine alkaloids on the performance of plant-parasitic and free-living nematodes. Pest Management Science. 65(7):823–830. https://doi.org/10.1002/ps.1764.
Tlak Gajger, I. and Dar, S. A. 2021. Plant allelochemicals as sources of insecticides. Insects. 12(3):189. https://doi.org/10.3390/insects12030189.
Tucuch-Haas, J. I.; Silva-Aguayo, G. and Rodríguez-Maciel, J. C. 2020. Oviposition of Bactericera cockerelli (Sulc) (Hemiptera: triozidae) on Capsicum chinense (Jacq) treated with spiromesifen or spirotetramat. Revista Fitotecnia Mexicana. 43(3):317-323. https://doi.org/10.35196/rfm.2020.3.317.
Vega, G. M. T.; Rodríguez, J. C.; Díaz, G. O.; Bujanos, M. R.; Mota, S. D.; Martínez, C. J. L.; Lagunes, T. A. y Garzón, T. A. 2008. Susceptibilidad a insecticidas en dos poblaciones mexicanas del salerillo, Bactericera cockerelli (Sulc) (Hemiptera: triozidae). Agrociencia. 42(4):463-471. https://www.scielo.org.mx/pdf/agro/v42n4/v42n4a9.pdf.
Venkatesh, G. and Arivudainambi, S. 2024. Efficacy of solvent extracts of Crotalaria paniculata Willd. (Fabaceae) and Holoptelea integrifolia Planch. (Ulmaceae) against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). International Journal of Entomology Research. 9(11):139-144. https://www.entomologyjournals.com/assets/archives/2024/vol9issue11/9327.pdf.
Walker, P. W.; Allen, G. R.; Tegg, R. S.; White, L. R. and Wilson, C. R. 2015. The tomato potato psyllid, Bactericera cockerelli (Šulc, 1909) (Hemiptera: triozidae): a review of the threat of the psyllid to Australian solanaceous crop industries and surveillance for incursions in potato crops. Austral Entomology. 54(6):339-349. https://doi.org/10.1111/AEN.12129.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Revista Mexicana de Ciencias Agrícolas

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores(as) que publiquen en Revista Mexicana de Ciencias Agrícolas aceptan las siguientes condiciones:
De acuerdo con la legislación de derechos de autor, Revista Mexicana de Ciencias Agrícolas reconoce y respeta el derecho moral de los autores(as), así como la titularidad del derecho patrimonial, el cual será cedido a la revista para su difusión en acceso abierto.
Los autores(as) deben de pagar una cuota por recepción de artículos antes de pasar por dictamen editorial. En caso de que la colaboración sea aceptada, el autor debe de parar la traducción de su texto al inglés.
Todos los textos publicados por Revista Mexicana de Ciencias Agrícolas -sin excepción- se distribuyen amparados bajo la licencia Creative Commons 4.0 atribución-no comercial (CC BY-NC 4.0 internacional), que permite a terceros utilizar lo publicado siempre que mencionen la autoría del trabajo y a la primera publicación en esta revista.
Los autores/as pueden realizar otros acuerdos contractuales independientes y adicionales para la distribución no exclusiva de la versión del artículo publicado en Revista Mexicana de Ciencias Agrícolas (por ejemplo incluirlo en un repositorio institucional o darlo a conocer en otros medios en papel o electrónicos) siempre que indique clara y explícitamente que el trabajo se publicó por primera vez en Revista Mexicana de Ciencias Agrícolas.
Para todo lo anterior, los autores(as) deben remitir el formato de carta-cesión de la propiedad de los derechos de la primera publicación debidamente requisitado y firmado por los autores(as). Este formato debe ser remitido en archivo PDF al correo: revista_atm@yahoo.com.mx; revistaagricola@inifap.gob.mx.
Esta obra está bajo una licencia de Creative Commons Reconocimiento-No Comercial 4.0 Internacional.


