Antifungal effect of pirul essential oil against phytopathogenic fungi of corn
DOI:
https://doi.org/10.29312/remexca.v16i5.3767Keywords:
distillation, fungicide, inhibitionAbstract
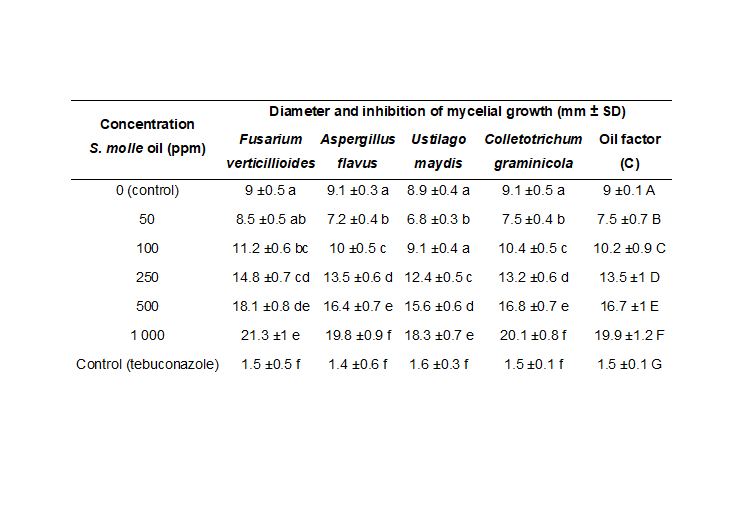
The present study evaluated the efficacy of pirul essential oil in the control of corn pathogens, including those responsible for significant diseases of this crop. Essential oil was extracted from fresh pirul leaves using the steam distillation method, obtaining a yield of 0.5%. The oil obtained was analyzed using the GC-MS technique, identifying main compounds, such as α-pinene, β-pinene, and D-limonene, known for their antimicrobial properties. Disc diffusion and cell viability assays showed dose-dependent inhibition of mycelial growth and a significant reduction in pathogen viability with increasing essential oil concentration. At a concentration of 1 000 ppm, the relative viability of the pathogens decreased to less than 10%, demonstrating potent antifungal activity. Comparatively, the control with tebuconazole showed a very low relative viability (1.5%), confirming the superiority of pirul essential oil as a natural antifungal agent. These results suggest that pirul essential oil is a promising and sustainable alternative to synthetic fungicides for the control of corn pathogens, contributing to safer and greener agricultural practices.
Downloads
References
Achar, P. N. and Sreenivasa, M. Y. 2021. Current perspectives of biocontrol agents for management of Fusarium verticillioides and its fumonisin in cereals A review. Journal of Fungi. 7(9):776-786. https://doi.org/10.3390/jof7090776.
Amaike, S. and Keller, N. P. 2011. Aspergillus flavus. Annual review of phytopathology. 49(1):107-133. https://doi.org/10.1146/annurev-phyto-072910-095221.
Božović, M.; Navarra, A.; Garzoli, S.; Pepi, F. and Ragno, R. 2017. Esential oils extraction: a 24-hour steam distillation systematic methodology. Natural Product Research. 31(3):2387-2396. https://doi.org/10.1080/14786419.2017.1309534.
Burt, S. 2004. Essential oils: their antibacterial properties and potential applications in foods a review. International Journal of Food Microbiology. 94(3):223-253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022.
Falcão, S. I.; Vilas-Boas, M.; Estevinho, L. M.; Barros, C.; Domingues, M. R. and Cardoso, S. M. 2010. Phenolic characterization of northeast Portuguese propolis: usual and unusual compounds. Analytical and Bioanalytical Chemistry. 396:887-897. https://doi.org/10.1007/s00216-009-3232-8.
FAO. 2021. The state of food and agriculture. Making agrifood systems more resilient to shocks. Food and Agriculture Organization of the United Nations. https://www.fao.org/publications/sofa/2021/en/.
Fisher, K. and Phillips, C. 2008. Potential antimicrobial uses of essential oils in food: is citrus the answer? Trends in Food Science & Technology. 19(3):156-164. https://doi.org/10.1016/j.tifs.2007.11.006.
Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J. Z.; Xie, X. Q. and Zimmer, A. 2008. Beta-caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences. 105(26):9099-9104. https://doi.org/10.1073/pnas.0803601105.
Goulson, D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology. 50(4):977-987. https://doi.org/10.1111/1365-2664.12111.
Guimarães, A. C.; Meireles, L. M.; Lemos, M. F.; Guimarães, M. C. C.; Endringer, D. C.; Fronza, M. and Scherer, R. 2019. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 24(13):2471-2479. https://doi.org/10.3390/molecules24132471.
Han, L.; Kong, X.; Xu, M. and Nie, J. 2021. Repeated exposure to fungicide tebuconazole alters the degradation characteristics, soil microbial community and functional profiles. Environmental pollution. 287:117660. https://doi.org/10.1016/j.envpol.2021.117660.
Hernández, A.; Estrada, B.; Rodríguez, R.; García, J. M.; Patiño, S. A. y Osorio, E. 2019. Importance of biological control of pests in corn (Zea mays L.). Revista Mexicana de Ciencias Agrícolas. 10(4):803-813. https://doi.org/10.29312/remexca.v10i4.1665.
Kazan, K. y Gardiner, D. M. 2018. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects. Molecular plant pathology. 19(7):1547-1562. https://doi.org/10.1111/mpp.12639.
Kohiyama, C. Y.; Ribeiro, M. M. Y.; Mossini, S. A. G.; Bando, E.; Silva-Bomfim, N.; Nerilo, S. B. and Machinski, M. 2015. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chemistry. 173:1006-1010. https://doi.org/10.1016/j.foodchem.2014.10.135.
Martínez, A.; Garcı́a, M. and Gold, S. 2002. The Ustilaginales as plant pests and model systems. Fungal Genetics and Biology. 35(1):1-20. https://doi.org/10.1006/fgbi.2001.1301.
Murrieta, U.; Medrano, J. M.; Guerra, D. y Valle, S. 2023. Composición de aceite esencial de hojas de Schinus molle L. afectada por el tiempo de extracción y escalamiento del proceso. Revista Chapingo Serie Ciencias Forestales y del Ambiente. 29(2):25-40. https://doi.org/10.5154/r.rchscfa.2022.04.027.
Olvera, G.; Machuca, R.; Borja, A.; Corona, A.; Zaragoza, I.; Arreola, J. G.; y Jiménez, J. 2021. Xilotecnia de la madera de Schinus molle L. de una plantación forestal comercial en Hidalgo, México. Madera y Bosques. 27(1):1-9. https://doi.org/10.21829/myb.2021.2711567.
Perczak, A.; Gwiazdowska, D.; Gwiazdowski, R.; Juś, K.; Marchwińska, K. and Waśkiewicz, A. 2019. The inhibitory potential of selected essential oils on Fusarium spp. growth and mycotoxins biosynthesis in maize seeds. Pathogens. 9(1):23-31. https://doi.org/10.3390/pathogens9010023.
Pimentel, D. and Burgess, M. 2014. Environmental and economic costs of the application of pesticides primarily in the United States Integrated pest management. In Integrated Pest Management. 47-71 pp. https://doi.org/10.1007/s10668-005-7314-2.
Prado, A. C.; Garces, H. G.; Bagagli, E.; Rall, V. L. M.; Furlanetto, A.; Fernandes Junior, A. and Furtado, F. B. 2019. Schinus molle essential oil as a potential source of bioactive compounds: antifungal and antibacterial properties. Journal of applied microbiology. 126(2):516-522. https://doi.org/10.1111/jam.14157.
Rey, C.; Pérez, K.; Guzmán, L. L. and Valarezo, J. E. 2018. Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae). Experimental and Applied Acarology. 76(3-4):399-411. https://doi.org/10.1007/s10493-018-0303-6.
Ruiz, J. R. y Salazar, M. E. 2021. Composición química y actividad antibacteriana de los aceites esenciales de Citrus paradisi, Juglans neotropica Diels, Schinus molle y Tagetes elliptica Smith. Revista de la Sociedad Química del Perú. 87(3):228-241. http://dx.doi.org/10.37761/rsqp.v87i3.350.
Tian, F.; Woo, S. Y.; Lee, S. Y.; Park, S. B.; Zheng, Y. and Chun, H. S. 2022. Antifungal activity of essential oil and plant-derived natural compounds against Aspergillus flavus. Antibiotics. 11(12):1727-1733. https://doi.org/10.3390/antibiotics11121727.
Tsushima, A. and Shirasu, K. 2022. Genomic resources of Colletotrichum fungi: development and application. Journal of General Plant Pathology. 88(6):349-357. https://doi.org/10.1007/s10327-022-01097-y.
Turchetti, G.; Garzoli, S.; Laghezza Masci, V.; Sabia, C.; Iseppi, R.; Giacomello, P. and Ovidi, E. 2020. Antimicrobial testing of Schinus molle (L.) leaf extracts and fractions followed by GC-MS investigation of biological active fractions. Molecules. 25(8):1977-1785. https://doi.org/10.3390/molecules25081977.
Volpini, A. F.; Lima, S. E.; Cardoso, C. A.; Cabral, M. R.; Louro, G. M.; Coutinho, E. J. and Simionatto, E. 2021. Chemical composition of essential oils from leaves and fruits of Schinus molle obtained by different extraction methods (hydrodistillation, fractional hydrodistillation and steam distillation) and seasonal variations. Journal of Essential Oil-Bearing Plants. 24(2):228-242. https://doi.org/10.1080/0972060X.2021.1914739.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


