Enzymatic activity in Sorghum bicolor by micro-nano encapsulated microbial metabolites and plant extracts
DOI:
https://doi.org/10.29312/remexca.v16i5.3755Keywords:
bioherbicides, nanotechnology, plant extracts, metabolitesAbstract
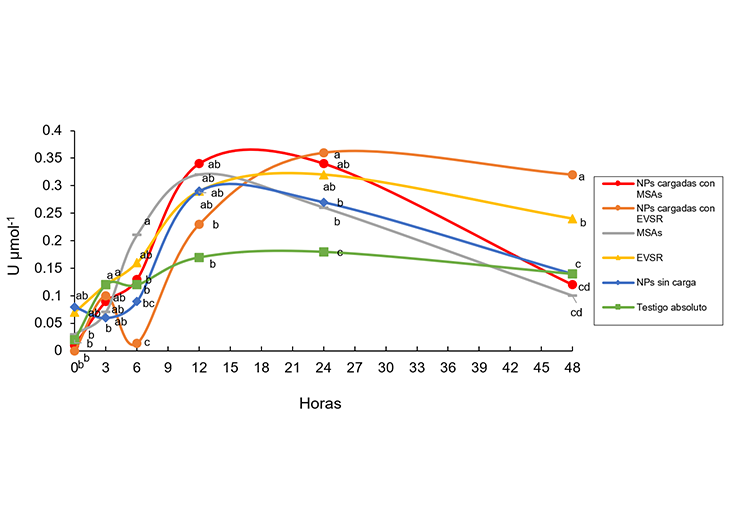
Chemical herbicides for weed control represent a current problem, since their indiscriminate use causes the emergence of resistant weed populations, in addition to affecting the environment and human health. Therefore, secondary metabolites of microorganisms and plant extracts in micro-nano encapsulated formulations emerge as a possible alternative to the use of chemically synthesized herbicides. Therefore, elucidating their mechanism of action is necessary to understand the biochemical changes they induce in plants and to develop weed control strategies. The objective of the research was to determine the activity of the antioxidant enzymes phenylalanine ammonia lyase, peroxidase, and superoxide dismutase in Sorghum bicolor plants treated with secondary metabolites of Alternaria sp. The secondary metabolites of microorganisms and a plant extract of Solanum rostratum, alone and formulated in micro-nano encapsulated formulations based on the biopolymer’s alginate and chitosan. The study was carried out during the month of June 2024, for this, S. bicolor y plants were used and the activity of the enzymes was determined for 0, 3, 6, 12, 24 and 48 h. It was observed that the formulations loaded with the Solanum rostratum plant extract and the secondary metabolites of microorganisms were those that induced the highest enzymatic activity at different times, reaching 0.36 and 0.34 U mol-1 respectively in the case of PAL, 4.7 and 4.3 U mol-1 with the peroxidase enzyme and 7.3 and 6.5 U mol-1 with super oxide dismutase. It is concluded that the secondary metabolites of microorganisms and the Solanum rostratum plant extract formulated in micro-nano encapsulates have potential as agents that can modify biochemical processes in plants.
Downloads
References
Alves, C.; Costa, E.; Sofiatti, J. R.; Forte, C. T.; Winter, F. L.; Holz, C. M. and Galon, L. 2018. Effect of herbicides on the oxidative stress in crop winter species. Anais da Academia Brasileira de Ciências. 90(02):1533-1542. https://doi.org/10.1590/0001-3765201820170482.
Anwar, S.; Naseem, S.; Karimi, S.; Asi, M. R.; Akrem, A. and Ali, Z. 2021. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat Way forward to lower the risk of synthetic herbicides. Frontiers in Plant Science. 12:632-390 https://doi.org/10.3389/fpls.2021.632390.
Ascacio-Valdés, J. A.; Aguilera-Carbó, A. F.; Buenrostro, J. J.; Prado-Barragán, A.; Rodríguez-Herrera, R. and Aguilar, C. N. 2016. The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. Journal of Basic Microbiology. 56(4):329-336. https://doi.org/10.1002/jobm.201500557.
Balah, M. A. 2020. Weed control ability of Egyptian natural products against annual, perennial and parasitic weeds. Acta Ecologica Sinica. 40(6):492-499. https://doi.org/10.1016/j.chnaes.2020.10.005.
Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart Jr, C. N. and Vargas, L. 2019. Defenses against ROS in crops and weeds: The effects of interference and herbicides. International journal of molecular sciences. 20(5):1086. https://doi.org/10.3390/ijms20051086.
Fancy, N. N.; Bahlmann, A. K. and Loake, G. J. 2017. Nitric oxide functions in plant abiotic stress. Plant, Cell & Environment. 40(4):462-472. https://doi.org/10.1111/pce.12707.
Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S. and Evidente, A. 2021. Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants. 10(3):543. https://doi.org/10.3390/plants10030543.
González-Gallegos, E.; Laredo-Alcalá, E.; Ascacio-Valdés, J.; Rodríguez, D. J. and Hernández-Castillo, F. D. 2015. Changes in the production of salicylic and jasmonic acid in potato plants (Solanum tuberosum) as response to foliar application of biotic and abiotic inductors. American Journal of Plant Sciences. 6(11):1785. https://doi.org/10.4236/ajps.2015.611179.
Grewal, S. K.; Gill, R. K.; Virk, H. K. and Bhardwaj, R. D. 2022. Methylglyoxal detoxification pathway-explored for the first time for imazethapyr tolerance in lentil (Lens culinaris L.). Plant Physiology and Biochemistry. 177:10-22. https://doi.org/10.1016/j.plaphy.2022.02.007.
Huang, H.; Ullah, F.; Zhou, D. X.; Yi, M. and Zhao, Y. 2019. Mechanisms of ROS regulation of plant development and stress responses. Frontiers in Plant Science. 10:800. https://doi.org/10.3389/fpls.2019.00800.
Husic, L.; Parić, A. and Mesic, A. 2023. Allelopathic and toxicological effects of Origanum vulgare L. essential oil. Caryologia. 76(1):97-102. https://doi.org/10.36253/caryologia-2132.
Kausar, T.; Jabeen, K.; Javaid, A. and Iqbal, S. 2022. Herbicidal efficacy of culture filtrates of Alternaria brassicicola and Alternaria gaisen against parthenium weed. Advances in Weed Science. 40:e02224640. https://doi.org/10.51694/AdvWeedSci/2022;40:00002.
Kumar, R.; Kumari, V. V.; Gujjar, R. S.; Kumari, M.; Goswami, S. K.; Datta, J. and Hossain, A. 2024. Evaluating the imazethapyr herbicide mediated regulation of phenol and glutathione metabolism and antioxidant activity in lentil seedlings. Peer J. 12:e16370. https://doi.org/10.7717/peerj.16370.
Nohatto, M. A.; Agostinetto, D.; Langaro, A. C.; Oliveira, C. D. and Ruchel, Q. 2016. Antioxidant activity of rice plants sprayed with herbicides. Pesquisa Agropecuária Tropical. 46(1):28-34. https://doi.org/10.1590/1983-40632016v4638011.
Ofosu, R.; Agyemang, E. D.; Márton, A.; Pásztor, G.; Taller, J. and Kazinczi, G. 2023. Herbicide resistance: managing weeds in a changing world. Agronomy. 13(6):1595. https://doi.org/10.3390/agronomy13061595.
Rodríguez-Pedroso, A. T.; Ramírez-Arrebato, M. Á.; Cárdenas-Travieso, R. M.; Falcón-Rodríguez, A. y Bautista-Baños, S. 2006. Efecto de la quitosana en la inducción de la actividad de enzimas relacionadas con la defensa y protección de plántulas de arroz (Oryza sativa L.) contra Pyricularia grisea Sacc. Revista Mexicana de Fitopatología. 24(1):1-7.
Romero-Tejeda, M.; Martínez-Damián, M. T. and Rodríguez-Pérez, J. E. 2015. Effect of storage temperature on enzyme activity and antioxidant capacity in Salvia officinalis L. shoots. Revista Chapingo Serie Horticultura. 21(3):199-213. https://doi.org/10.5154/r.rchsh.2015.01.003.
Sinegovskaya, V. and Dushko, O. 2021. Role of enzyme activity in increasing soybean plants’ resistance to herbicides. In: E3S Web of Conferences. 254:02007. EDP Sciences. https://doi.org/10.1051/e3sconf/202125402007.
Taban, A.; Saharkhiz, M. J. y Kavoosi, G. 2021. Development of pre-emergence herbicide based on Arabic gum-gelatin, apple pectin and savory essential oil nano-particles: a potential green alternative to metribuzin. International Journal of Biological Macromolecules. 167:756-765. https://doi.org/10.1016/j.ijbiomac.2020.12.007.
Todero, I.; Confortin, T. C.; Luft, L.; Brun, T.; Ugalde, G. A.; Almeida, T. C. and Mazutti, M. A. 2018. Formulation of a bioherbicide with metabolites from Phoma sp. Scientia Horticulturae. 241:285-292. https://doi.org/10.1016/j.scienta.2018.07.009.
Traxler, C.; Gaines, T. A.; Küpper, A.; Luemmen, P. y Dayan, F. E. 2023. The nexus between reactive oxygen species and the mechanism of action of herbicides. Journal of Biological Chemistry. 105-267 pp. https://doi.org/10.1016/j.jbc.2023.105267.
Tucuch-Pérez, M. A.; Mendo-González, E. I.; Ledezma-Pérez, A.; Iliná, A.; Hernández-Castillo, F. D.; Barrera-Martinez, C. L. and Arredondo-Valdés, R. 2023. The herbicidal activity of nano-and microencapsulated plant extracts on the development of the indicator plants Sorghum bicolor and Phaseolus vulgaris and their potential for weed control. Agriculture. 13(11):2041. https://doi.org/10.3390/agriculture13112041.
Ureña-Saborío, H.; Madrigal-Carballo, S.; Sandoval, J.; Vega-Baudrit, J. R. and Rodríguez-Morales, A. 2017. Encapsulation of bacterial metabolic infiltrates isolated from different Bacillus strains in chitosan nanoparticles as potential green chemistry-based biocontrol agents against Radopholus similis. Journal of Renewable Materials. 5(3-4):290-299. https://doi.org/10.7569/JRM.2017.634119.
Van Bruggen, A. H.; Finckh, M. R.; He, M.; Ritsema, C. J.; Harkes, P.; Knuth, D. and Geissen, V. 2021. Indirect effects of the herbicide glyphosate on plant, animal and human health through its effects on microbial communities. Frontiers in Environmental Science. 9:763-917. https://doi.org/10.3389/fenvs.2021.763917.
Yedidia, I.; Benhamou, N. and Chet, I. 1999. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Applied and environmental microbiology. 65(3):1061-1070. https://doi.org/10.1128/AEM.65.3.1061-1070.1999.
Yin, X. L.; Jiang, L.; Song, N. H. and Yang, H. 2008. Toxic reactivity of wheat (Triticum aestivum) plants to herbicide isoproturon. Journal of agricultural and food chemistry. 56(12):4825-4831. https://doi.org/10.1021/jf800795v.
Zabot, G. L.; Schaefer-Rodrigues, F.; Polano-Ody, L.; Vinícius-Tres, M.; Herrera, E.; Palacin, H. and Olivera-Montenegro, L. 2022. Encapsulation of bioactive compounds for food and agricultural applications. Polymers. 14(19):4194. https://doi.org/10.3390/polym14194194.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


