Potential weeds as a trap crop for Meloidogyne enterolobii and Nacobbus aberrans
DOI:
https://doi.org/10.29312/remexca.v15i8.3610Keywords:
Dysphania ambrosioides, Malva parviflora, Oxalis corniculata, Portulaca oleracea, Tagetes erectaAbstract
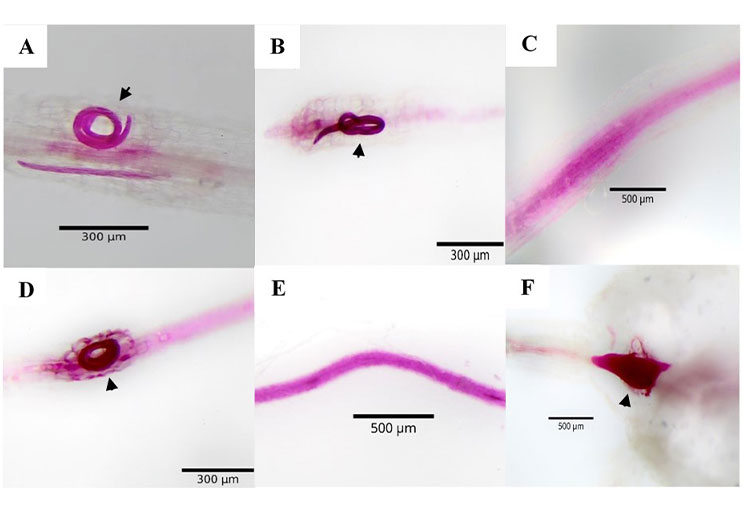
The use of weeds as an agroecological management strategy for phytonematodes has gained importance due to their implementation as trap plants that interfere with their biological cycle; therefore, this research aimed to evaluate the percentage of reproduction of Meloidogyne enterolobii and Nacobbus aberrans in five weeds. This experiment was carried out at the College of Postgraduates, Montecillo Campus, State of Mexico, Mexico, in 2023. The chili genotype CM-334 (control) was used as a susceptibility reference and each experimental unit was inoculated with 1000 J2 of each nematode species. The response variables were galling, egg masses, eggs, number of females and juveniles per g of root at 35 days after inoculation (dai) for Meloidogyne enterolobii and 45 dai for Na. A completely randomized experimental design with factorial arrangement was used. The weeds Tagetes erecta, Portulaca oleracea, Dysphania ambrosioides, Malva parviflora, and Oxalis corniculata showed a 100% decrease in the number of galls, egg masses, and eggs per g of root for Nacobbus aberrans, compared to the control. These last two parameters were similar for Meloidogyne enterolobii. All weeds evaluated showed a differential reproduction percentage for both nematodes in the number of females and individuals per g of root (7.34-100%). The results obtained indicate that these weeds can be used as a potential trap crop for the management of the nematodes Meloidogyne enterolobii and Nacobbus aberrans.
Downloads
References
Byrd, Jr. R.; Kirkpatrick, T. and Barker, K. R. 1983. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology. 15(1):142-143.
Cid del Prado, V. I.; Franco, F. N. and Godinez, D. V. 2018. Plant parasitic nematodes and management strategies of major crops in Mexico. In: plant parasitic nematodes in sustainable agriculture of North America, sustainability in plant and crop protection. Subbotin, S. A and Chitambar, J. J Ed. Springer Nature. Switzerland. 31-68 pp.
Cid del Prado, V. I.; Tovar, S. A. y Hernández, J. A. 2001. Distribución de especies y razas de Meloidogyne en México. Revista Mexicana de Fitopatología. 19(1):32-39.
Cook, R. and Starr, J. L. 2006. Resistant cultivars. In: plant nematology. Perry, R. and Moens, M. Ed. CABI. UK. 370-391 pp.
Ferraz, S. y Valle, L. A. 1997. Controle de fitonematoides por plantas antagónicas. Viçosa, MG. Ed. UFV. 73 p.
Filialuna, O.; Wram, C. and Zasada, I. 2022. What is the optimal way to assess Meloidogyne spp. reproduction in greenhouse pot experiments? Journal of Nematology. 54(1):1-9. Doi: 10.2478/jofnem-2022-0012.
Groover, W.; Lawrence, K. S. and Donald, P. 2019. Reproductive rate differences of root-knot nematodes from multiple crops in a single field. Nematropica. 49(2):152-156.
Hussey, R. S. and Janssen, G. J. W. 2004. Root-knot nematode: Meloidogyne species. In: plant resistence to parasitic nematodes. Starr, J. L.; Cook, R. and Bridge, J. Ed. CABI Publishing, New York. 43-70 pp.
Hussey, R. S. and McGuire, J. M. 1987. Interaction with other organisms. In: principles and practice of nematode control in crops. Brown, R. H. and Kerry, B. R. Ed. Academic Press. Australia. 293-328 pp.
Kanchan, B. M.; Jayanthi, M.; Shivani, C.; Uma, R. and Pranab, K. M. 2023. In planta transformation of Polianthes tuberosa for concomitant knockdown of flp-1, flp-12 and flp-18 genes induced root-knot nematode resistance. Scientia Horticulturae. 311(1):1-13. Doi: 10.1016/j.scienta.2022.111764.
Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M. and Siddiqui, M. A. 2019. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: in vitro and greenhouse study. Curr. Plant Biol. 20:100115. Doi: 10.1016/j.cpb.2019.100115.
Kirwa, H. K.; Murungi, L. K.; Beck, J. J. and Torto, B. 2018. Elicitation of differential responses in the root-knot nematode Meloidogyne incognita to tomato root exudate cytokinin, flavonoids, and alkaloids. Journal of Agricultural and Food Chemistry. 66(43):11291-11300. Doi: 10.1021/acs.jafc.8b05101.
Manzanilla, L. R.; Costilla, M. A.; Doucet, M.; Franco, J.; Inserra, R. N.; Lehman, P. S.; Cid Del Prado, V. I.; Souza, R. M. and Evans, K. 2002. The genus Nacobbus Thorne and Allen, 1944 (Nematoda: Pratylenchidae): systematics, distribution, biology and management. Nematropica. 32(2):149-227.
Moens, M.; Perry, R. N. and Starr, J. L. 2009. Meloidogyne species is a diverse group of novel and important plant parasites. In: root-knot nematodes. Perry, R. N.; Moens, M. and Starr, J. L. Ed. CAB International. Wallingford. UK. 1-17 pp.
Moreira, F. J.; Santos, C. D.; Innecco, R. y Silva, G. S. 2015. Control alternativo de nematoide das galhas (Meloidogyne incognita) raça 2, con óleos esenciales en solo. Summa Phytopathologica Botucatu. 41(3):207-213.
Ntidi, K. N.; Fourie, H. and Daneel, M. S. 2016. Greenhouse and field evaluations of commonly occurring weed species for their host suitability to Meloidogyne species. International Journal of Pest Management 62(1):11-19. Doi: 10.1080/09670874.2015.1087602.
Oostenbrink, M. 1966. Major characteristics of the relation between nematodes and plant. Wageningen University y Research. Netherlands. 1-46 pp.
Proita, K.; Carneiro, R.; Falcão, R.; Gomes, A.; Leal-Bertioli, S.; Guimarães, P. and Bertioli, D. 2008. Post-infection development and histopathology of Meloidogyne arenaria race 1 on Arachis spp. Plant Pathology. 57(5):974-980. Doi: 10.1111/j.1365-3059.2008.01861.x.
Rich, J. R.; Brito, J. A.; Kaur, R. and Ferrell, J. A. 2009. Weed species as hosts of Meloidogyne: a review. Nematropica. 39(2):157-185.
SAS Institute. 2012. SAS/STAT® 9.3 User’s Guide. SAS Institute.
Sato, K.; Kadota, Y. and Shirasu, K. 2019. Plant immune responses to parasitic nematodes. Frontiers in Plant Science. 10(2):1-14. Doi: 10.3389/fpls.2019.01165.
SIAP. 2019. Servicio de Información Agropecuaria y Pesquera. Anuario estadístico de la producción agrícola. Secretaría de Agricultura y Desarrollo Rural (SADER). Ciudad de México. https://nube.siap.gob.mx/cierreagricola/.
Triviño, G. C. 2004. Tecnología biológica para el manejo del nematodo agallador de raíces Meloidogyne spp. en tomate. Boletín técnico (109). Estación Experimental Boliche, Guayaquil. Ecuador. 15 p.
Uddin, K. M.; Shukor, J. A.; Hossain, S. M.; Altaf, N. M.; Eaqub, A. M. and Rahman, M. M. 2014. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. The Scientific World Journal. 23(2):1-6. Doi: 10.1155/2014/951019.
Villar-Luna, E.; García, E. J.; Gómez, R. O.; Rojas, M. R. I. y Zavaleta, M. E. 2015. Defense gene expression in root galls induced by Nacobbus aberrans in CM334 chilli plants. Helminthologia. 52(1):77-82.
Villar-Luna, E.; Gómez, R. O.; Rojas, M. R. I. and Zavaleta, M. E. 2016. Presence of Meloidogyne enterolobii on jalapeño pepper (Capsicum annuum L.) in Sinaloa, México. Helminthologia. 53(2):155-160.
Vrain, T. C. 1977. A technique for the collection of larvae of Meloidogyne spp. and a comparison of eggs and larvae as inocula. Journal of Nematology. 9(3):49-51.
Wuyts, N.; Swennen, R. and De Wael, D. 2006. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behavior of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology. 8(1):89-101. Doi:10.1163/156854106776179953.
Yang, G.; Zhou, B.; Zhang, X.; Zhang, Z.; Wu, Y.; Zhang, Y.; Lü, S.; Zou, Q.; Gao, Y. and Teng, L. 2016. Effects of tomato root exudates on Meloidogyne incognita. Plos One. 11(4):1-16. Doi: 10.1371/journal.pone.0154675.
Zhou, Y.; Xin, H.; Rahman, K.; Wang, S.; Peng, C. and Zhang, H. 2015. Portulaca oleracea L.: a review of phytochemistry and pharmacological effects. BioMed Research International. 4:1-11. Doi: 10.1155/2015/925631.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


