Biological pest control in Mexican agriculture
DOI:
https://doi.org/10.29312/remexca.v13i27.3251Keywords:
biological control, entomopathogens, parasitoids, plant extracts, plant hormones, predators, repellent plants, volatile compoundsAbstract
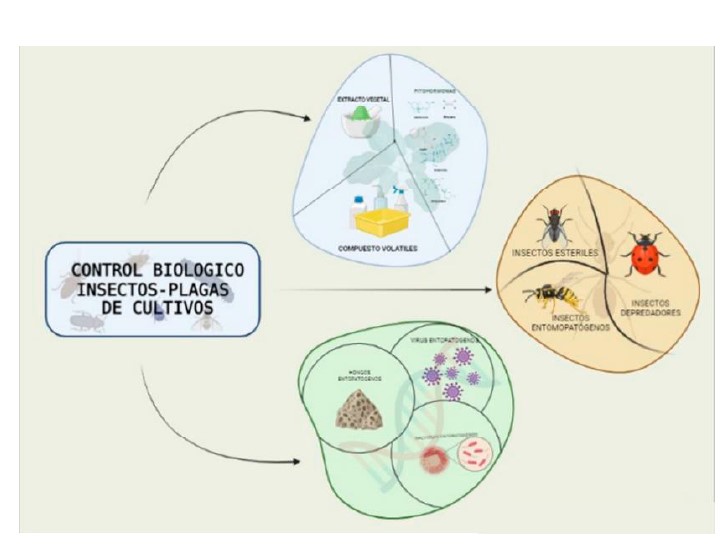
Due to the continuous increase of the human population, the demand for food production should increase 70-100% in the following years. However, the food security of humanity is affected by several factors, among them pest insects, which are currently controlled through the application of large doses of synthetic insecticides, which generate serious problems in human health, resistance to pests, residues in foods, environmental pollution, outbreaks of secondary pests and reduction in populations of beneficial insects. Given this global scenario, these problems generate a greater demand for pest control methods that are efficient and environmentally friendly, so the objective of the present work was to address in a synthetic way the development and progress of research conducted in Mexico on the biological control of pest insects. This review focuses on technologies with a solid ecological basis for the gradual restoration of biodiversity lost in agroecosystems, so promising alternatives for pest control of great relevance in the Mexican countryside are addressed, such as the use of beneficial insects such as parasitoids, predators and entomopathogens that cause the death of pest insects, the use of sterile insect, bioinsecticides, such as microbial pesticides and other entomopathogens, incorporated plant protectors and biochemical pesticides. The demand for techniques related to the biological control of pest insects in Mexico should address the problems of transboundary, exotic, newly introduced pests and those pests that have remained for several decades in the country.
Downloads
References
Abdullahi, A. M.; Sarki, A.; Hafizu, M. S.; Kunihya, I. Z.; Kolawole, A. A.; Nassai, I. and Haruna, M. Y. 2019 Phyto-chemicals of some plant leaf powder as anti-insect agents against maize weevils Sitophilus zeamais (Coleoptera: Curculionidae). Fudma J. Sci. 3(4):291-295. Alarcón, R.; Guzmán, T.; Penieres, J. y Navarrete, R. 2016. Actividad repelente e insecticida de hojas, flores y extractos de llama del bosque (Spathodea campanulata B.), en gorgojos de granos almacenados (Sitophilus zeamais M.). La Calera. 16(27):94-99. https://doi.org/ 10.5377/calera.v16i27.6009. DOI: https://doi.org/10.5377/calera.v16i27.6009
Altieri, M. y Nicholls, C. 2008. Optimizando el manejo agroecológico de plagas a través de la salud del suelo. Agroecología. 1:29-36.
Ángeles, L. Y. I.; Martínez, G. N. A.; Ramírez, R. R.; López, M. G.; Sánchez, H. C. and Délano, F. J. P. 2012. Cross-Kingdom effects of plant-plant signaling via volatile organic compounds emitted by tomato (Solanum lycopersicum) plants infested by the greenhouse whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 38(11):1376-1386. https://doi.org/ 10.1007/s10886-012-0201-z. DOI: https://doi.org/10.1007/s10886-012-0201-z
Anguelov, R.; Dumont, Y. and Yatat, D. I. V. 2020. Sustainable vector/pest control using the permanent sterile insect technique. Math. Methods Appl. Sci. 43(18):10391-10412. https://doi.org/10.1002/mma.6385.
Arredondo, B. H. C. y Rodríguez, B. L. A. 2020. Programas de control biológico de México. In: Arredondo-Bernal, H. C.; Tamayo, M. F. and Rodríguez-del Bosque, L. A. (Ed.). Fundamento y práctica del control biológico de plagas y enfermedades. Biblioteca básica de agricultura (BBA). Cd. de México. 523-546 pp.
Bautista, L. A. and Espinosa, G. F. J. 2013. Odor uniformity among tomato individuals in response to herbivore depends on insect species. PLoS One 8(10):1-12. https://doi.org/10.1371/ journal.pone.0077199. DOI: https://doi.org/10.1371/journal.pone.0077199
Brechelt, A. 2004. El manejo ecológico de plagas y enfermedades. Red de acción en plaguicidas y sus alternativas para América Latina (RAP-AL). Fundación Agricultura y Medio Ambiente (FAMA). 1:14.
Carrillo, R. M. T. y Blanco, L. A. 2009. Potencial y algunos de los mecanismos de acción de los hongos entomopatógenos para el control de insectos plaga. Acta niversitaria. 19(2):40-49. Castillo, L.; Jiménez, J. y Delgado, M. 2010. Metabolitos secundarios de las familias Annonaceae, Solanaceae y Meliaceae utilizados como control biológico de insectos. Trop. Subtrop. Agroecosystems.12(3):445-462.
Cancino, J.; Ruiz, L.; López, E.; Aguilar, E.; Galvez, C.; Montoya, P. and Liedo, P. 2019. Suppression of Ceratitis capitata (Wied.) (Diptera: Tephritidae) populations in coffee in the Mexico-Guatemala border region through the augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biocontrol Sci. Technol. 29(8):822-826. https://doi.org/10.1080/09583157.2019.1608507.
Culliney, T. 2014. Crop losses to arthropods. In: Pimentel, D. and Peshin, R. (Ed.) Integrated pest management. Springer, Dordrecht. 201-225. https://doi.org/10.1007/978-94-007-7796-5-8. DOI: https://doi.org/10.1007/978-94-007-7796-5_8
Dimetry, N. Z. 2012. Prospects of botanical pesticides for the future in integrated pest management programme (IPM) with special reference to neem uses in Egypt. Arch. Phytopathol. Pflanzenschutz 45(10):1138-1161. https://doi.org/10.1080/03235408.2012.657932. DOI: https://doi.org/10.1080/03235408.2012.657932
Dong, F.; Fu, X.; Watanabe, N.; Su, X. and Yang, Z. 2016. Recent advances in the emission and functions of plant vegetative volatiles. Molecules. 21(2):1-10. https://doi.org/10.3390/ molecules21020124. DOI: https://doi.org/10.3390/molecules21020124
Eriksson, M.; Hardell, L.; Carlberg, M. and Kerman, M. 2008. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int. J. Cancer. 123(7):1657-1663. https://doi.org/10.1002/ijc.23589. FAO. 2018. Organización de las Naciones Unidas para la Alimentación y la Agricultura. El futuro de la alimentación y la agricultura: vías alternativas hacia el 2050. Versión resumida. Rome. 64 pp. http://www.fao.org/3/CA1553ES/ca1553es.pdf.
Felipe, V. M.; Talamas, E. J. and Sánchez, P. S. R. 2019. Scelionidae (Hymenoptera) parasitizing eggs of Bagrada hilaris (Hemiptera, Pentatomidae) in Mexico. J. Hymenopt. Res. 73:143. https://doi.org/10.3897/jhr.73.36654.
Gonçalves, G. L. P.; Lira, S. P.; Glssi, D. S. y Vendramim, J. D. 2021. Bioactividad de extractos de Solanaceae frente a Zabrotes subfasciatus. Acta Biol. Colomb. 26(1):62-71. https://doi.org/10.15446/abc.v26n1.84712.
Hendrichs, J. 2000. Use of the sterile insect technique against key insect pests. Sustain. Develop. I. 2:75-79.
Jaraleño, T. J.; Lomeli, F. J. R.; Rodríguez, L. E.; Bujanos, M. R. and Rodríguez, R. S. E. 2020. Egg parasitoids survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in maize and sorghum in Central Mexico. Insects. 11(3):157. https://doi.org/10.3390/ insects11030157.
Kim, D. S. 2021. A Review on the insecticidal activity of neem extracts (azadirachtin) and its current status of practical use in Korea. Korean J. Appl. Entomol. 60(4):463-471. https://doi.org/10.25085/rsea.800104. Lannacone, J. y Reyes, M. 2001. Efecto de la rotenona y neem sobre Bemisia tabaco Gennadius (Homoptera: Aleyrodidae) y Liriomyza huidobrensis Blanchard (Diptera: Agromyzidae) plagas del tomate en el Perú. Agronomía Trop. 51(1):65-79.
Marec, F. and Vreysen, M. J. 2019. Advances and challenges of using the sterile insect technique for the management of pest Lepidoptera. Insects. 10(11):371. https://doi.org/10.3390/ insects10110371.
Montoya, P.; López, P.; Cruz, J.; Cadena, C.; Cancino, J. and Liedo, P. 2017. Effect of Diachasmimorpha longicaudata releases on the native parasitoid guild attacking Anastrepha spp. larvae in disturbed zones of Chiapas, Mexico. BioControl. 62(5):581-593. https://doi.org/10.1007/s10526-017-9826-8. Ohyama, K.; Okawa, A.; Moriuchi, Y. and Fujimoto, Y. 2013. Biosynthesis of steroidal alkaloids in Solanaceae plants: Involvement of an aldehyde intermediate during C-26 amination. Phytochemistry. 89:26-31. https://doi.org/10.1016/j.phytochem.2013.01.010. Ordoñez, G. M.; Ríos, V. C.; Berlanga, R. D. I.; Acosta, M. C. H.; Salas, M. M. A. y Cambero, C. O. J. 2015. Reporte preeliminar de entomopatogenos del ‘gusano cogollero’ Spodoptera Frugiperda (Lepidoptera: Noctuidae) en Chihuahua, México. Entomol. Mex. 2:241-246. Orozco, S. M.; Robles, G. M.; Hernández, F. L. M.; Velázquez, M. J. J.; Jesús, B. G. M.; Manzanilla, R. M.; Manzo, S. G. y Nieto, A. D. 2016. Uso de aceites y extractos vegetales para el control de Diaphorina citri Kuwayama1 en lima mexicana en el Trópico Seco de México. Southwest. Entomol. 41(4):1051-1066. https://doi.org/10.3958/059.041.0405. Pacheco, H. M.; Reséndiz, M. J. y Arriola, P. V. J. 2019. Organismos entomopatógenos como control biológico en los sectores agropecuario y forestal de México: una revisión. Rev. Mex. Cienc. Forest. 10(56):4-32. https://doi.org/10.29298/rmcf.v10i56.496. Pavela, R. 2016. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects - a review. Plant Protect. Sci. 52(4):229-241. https://doi.org/10.17221/31/2016-PPS. DOI: https://doi.org/10.17221/31/2016-PPS
Pérez, T. B.; Aragón, G. A.; Cuate, M. V.; López, O. J. F.; Aragón, S. M. y Lugo, G. G. 2017. Efecto de la aplicación en campo de mezclas de extractos vegetales sobre la presencia y daños de insectos plaga en el cultivo de Amaranthus hypochondriacus L. Rev. Fac. Agron. 34:477-496.
Peterson, C. and Coats, J. 2001. Insect repellents-past, present and future. Pestic. Outlook. 12(4):154-158. https://doi.org/10.1039/b106296b. DOI: https://doi.org/10.1039/b106296b
Pizarro, D.; Silva, G.; Tapia, M.; Rodríguez, J. C.; Urbina, A.; Lagunes, A.; Santillán, O. C.; Robles, B. A. y Aguilar, M. S. 2013. Actividad insecticida del polvo de Peumus boldus Molina (Monimiaceae) contra Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). B. Latinoam. Caribe Pl. 12(4):420-430.
Ríos, V. C.; Pérez, C. D. A.; Salas, M. M. Á.; Berlanga, R. D. I.; Ornelas, P. J. J.; Muñiz, C. H. A.; Cambero, C. J. and Jacobo, C. J. L. 2014. Pathogenicity of the Hypocreales fungi Beauveria bassiana and Metarhizium anisopliae against insect pests of tomato. Southwest. Entomol. 39(4):739-750. https://doi.org/10.3958/059.039.0405. DOI: https://doi.org/10.3958/059.039.0405
Ruiz, J. K. Z.; Osorio, O. R.; Hernández, H. L. U.; Ochoa, F. A. A.; Silva, V. R. y Mendez, Z. G. 2021. Actividad acaricida de extractos de plantas contra el ácaro rojo de las palmeras Raoiella indica (Acari: Tenuipalpidae). Rev. Soc. Entomol. Arg. 80(1):33-39.
Somoza, V. C. E.; Hernández, V. V. M.; Peña, Ch. G.; Torres, G. G.; Huerta, P. A.; Ortega, M. L. D. and Salazar, M. J. A. 2018. Interaction of Beauveria bassiana strain HPI-019/14 and Bacillus thuringiensis strain GP139 for the biological control of Bemisia tabaci in strawberry. Bull. Insectology. 71(2):201-209.
Sun, W.; Shahrajabian, M. H. and Cheng, Q. 2020. Pyrethrum an organic and natural pesticide. J. Biol. Environ. Sci. 14(40):41-44.
Vreysen, M. J.; Abd, A. A. M.; Bourtzis, K.; Bouyer, J.; Caceres, C. X.; de Beer, C.; Oliveira, C. D. X.; Maiga, H.; Mamai, W.; Nikolouli, K.; Yamada, H. and Pereira, R. 2021. The insect pest control laboratory of the joint FAO/IAEA programme: ten years of research and development, achievements and challenges in support of the sterile insect technique. Insects. 12(4):346. https://doi.org/10.3390/insects12040346.
Wakeil, N. E. 2013. Botanical pesticides and their mode of action. Gesunde Pflanzen. 65(4):125-149. https://doi.org/10.1007/s10343-013-0308-3. DOI: https://doi.org/10.1007/s10343-013-0308-3
Wong, T. T. Y.; Ramadan, M. M.; McInnis, D. O.; Mochizuki, N.; Nishimoto, J. A. and Herr, J. C. 1991. Augmentative releases of Diachasmimorpha tryoni (Hymenoptera: Braconidae) to suppress a Mediterranean fruit fly (Diptera: Tephritidae) population in Kula, Maui, Hawaii. Biol. Control. 1(1):2-7. https://doi.org/10.1016/1049-9644(91)90094-G. DOI: https://doi.org/10.1016/1049-9644(91)90094-G
Zhang, P.; Qin, D.; Chen, J. and Zhang, Z. 2020. Plants in the genus Tephrosia: valuable resources for botanical insecticides. Insects.11(10):721. https://doi.org/10.3390/insects11100721.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


