Photosynthetic rate and biomass production by inoculation of Scleroderma sp. with different concentrations of NaCl in pecan tree
DOI:
https://doi.org/10.29312/remexca.v13i7.3024Keywords:
mycorrhizae, seedling behavior, stressful mediumAbstract
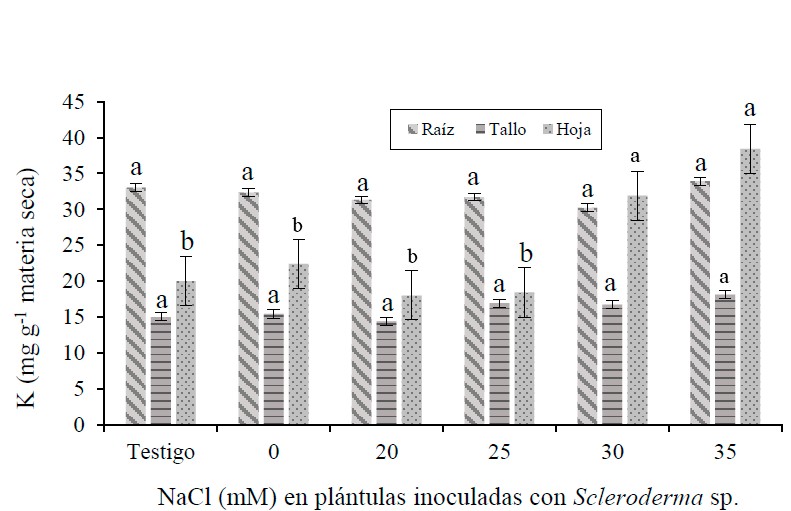
The pecan tree [Carya illinoinensis (Wangenh) K. Koch] is one of the fruit trees established in northern Mexico, where there is a considerable area of soils with different degrees of salinity, to which the tree is sensitive. In seedlings inoculated with Scleroderma sp., the effect of NaCl was evaluated at concentrations of 0, 20, 25, 30 and 35 mM, including a control without inoculation and without NaCl. The experiment was carried out at the University Regional Unit of Arid Zones in Bermejillo, Durango, Mexico, under greenhouse conditions. The seedlings were watered twice a week, during 2018. The plants inoculated and under non-saline conditions, showed the highest photosynthesis and transpiration, followed by the control seedlings. The concentration of Na+ in root, stem and leaf was lower in the control and in the inoculated seedlings and without NaCl. The concentration of K in inoculated seedlings and under saline and non-saline conditions was like the control. The k/Na ratio was higher in the control and in the inoculated seedlings and under non-saline conditions and decreased as NaCl concentration increased, particularly in the root. The control seedlings showed higher dry weight in root, stem and leaf than those inoculated with Scleroderma sp. and under salt stress, but it was lower than in those inoculated with the fungus and under non-saline conditions. In seedlings under saline stress of 20 and 35 mM, the decrease in dry weight was 8.5 and 47%, compared to the control. Walnut seedlings inoculated with Scleroderma sp. and under non-saline condition, they showed better physiological response and biomass accumulation and not under saline condition.
Downloads
References
Babuin, M.; Echeverria, M.; Menendez, A. and Maiale, S. 2016. Arbuscular mycorrhizal pecan seedlings alleviate effect of restricted water supply. HortScience. 51(3):212-215. Doi: https://doi.org/10.21273/hortsci.51.3.212.
Bandou, E.; Lebailly, F.; Muller, F.; Dulormne, M.; Toribio, A.; Chabrol, J.; Courtecuisse R.; Plenchette, C.; Prin, Y.; Duponnois, R.; Thiao, M.; Sylla, S.; Dreyfus, B. and Ba, A. 2006. The ectomycorrhizal fungus Scleroderma bermudense alleviates salt stress in seagrape (Coccoloba uvifera L.) seedlings. Mycorrhiza. 16(8):559-565. Doi: https://doi.org/10. 1007/s00572-006-0073-6.
Bassil, E.; Coku, A. and Blumwald, E. 2012. Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 63(16):5727-5740. Doi: https://doi.org/10.1093/jxb/ers250.
Behrozz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Saadat, S. and Leslie, Ch. 2019. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience. 54(6):1087-1092. Doi: https://doi.org/10.21273/HORTSCI13961-19.
Birhane, E.; Sterck, F. J.; Fetene. M.; Bongers, F. and Kuyper, T. 2012. Arbuscular mycorrhizal fungi enhance photosynthesis, water use effciency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia. 169(4):895-904. Doi: https://doi.org/10.1007/s00442-012-2258-3.
Bonito, G.; Brnneman, T. and Vilgalys, R. 2011. Ectomycorrhizal fungal diversity in orchards of cultivated pecan (Carya illinoinensis; Juglandaceae). Mycorrhiza. 21(7):601-612. Doi: https://doi.org/10.1007/s00572-011-0368-0.
Campos, V. A.; Arreola, A. J.; Trejo, C. R.; Borja, A.; López, S. y Hernández, R. 2017. Respuesta fisiológica, acumulación iónica y peso seco en portainjertos de nogal pecanero (Carya illinoinensis (wangenh) k. koch) desarrollados bajo condiciones de estrés salino. Interciencia. 42(11):744-749.
Chen, Z.; Zhou, M. X.; Newman, I. A.; Mendham, N. J.; Zhang, G. P. and Shabala, S. 2007. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 34(2):150-162. Doi: https://doi.org/10.1071/FP06237.
Estrada, B.; Aroca, R.; Barea, J. and Ruíz, L. J. M. 2013. Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Science. 201(1):42-51. Doi: https://doi.org/10.1016/j.plantsci.2012. 11.009.
Evelin, H.; Kapoor, R. and Giri, B. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann. Bot. 104(7):1263-1280. Doi: https://doi.org/10.1093/ aob/mcp251.
Feng, G.; Zhang, F.; Li, X.; Tian, C. T.; Tanq, C. and Rengel, Z. 2006. Uptake of nitrogen from indigenous soil pool by cotton plant inoculated with arbuscular mycorrhizal fungi. Comm. Soil Sci. Plant Analysis. 33(19):3825-3836. Doi: https://doi.org/10.1081/ CSS-120015925.
Giri, B.; Kapoor, R. and Mukerji, K. G. 2003. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils. 38(3):170-175. Doi: https://doi.org/10.1007/s00374-003-0636-z.
Giri, B.; Kapoor, R. and Mukerji, K. G. 2007. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum, may be partly related to elevated K/Na ratios in root and shoot tissues. Microbial Ecol. 54(4):753-760. Doi: https://doi.org/ 10.1007/s00248-007-9239-9. Guzmán, H. G.; Cortés, P. A.; Guzmán, D. L.; Ramírez, G. F. and Refugio, S. J. M. 2013. An emendation of Scleroderma, new records, and review of the known species in Mexico. Rev. Mex. Bio. 84(suplemento):S173-S191. Doi: https://doi.org/10.7550/rmb.31979.
Lee, S. H.; Calvo, P. M.; Chung, G. C. and Zwiazek, J. J. 2010. Role of aquaporins in root water transport of ectomycorrhizal jack pine (Pinus banksiana) seedlings exposed to NaCl and fluoride. Plant Cell Environ. 33(5):769-780. Doi: https://doi.org/10.1111/j.1365-3040.2009.02103.x
Li. J.; Bao, S.; Zhang, Y.; Ma, X.; Mishra, K. M.; Sun, J.; Sa, G.; Shen, X.; Polle, A. and Chen, S. 2012. Paxillus involutus strains MAJ and NAU mediate K+/Na+ homeostasis in ectomycorrhizal Populus×canescens under NaCl stress. Plant Physiol. Doi: https://doi.org/10.1104/pp.112.195370. 159(4): 1771-1786.
Lu, Y. W.; Wang, G. Q.; Meng, Q. J.; Zhang, W. H. and, Duan, B. L. 2014. Growth and physiological responses to arbuscular mycorrhizal fungi and salt stress in dioecious plant Populus tomentosa. Canadian J. Forest Res. 44(9):1020-1031. Doi: https://doi.org/10.1139/ cjfr-2014-0009.
Ma, X.; Sun, M.; Sa, G.; Zhang, Y.; Li, J.; Sun, J.; Shen, X.; Polle, A. and Chen, S. 2014. Ion fluxes in Paxillus involutus-inoculated roots of Populus×canescens under saline stress. 108(4):99-108. Doi: https://doi.org/10.1016/j.envexpbot.2013.11.016.
Miyamoto, S. and Nesbitt, M. 2011. Effectiveness of soil salinity management practices in basin irrigated pecan orchards. HortTechnology. 21(5):569-576. Doi: https://doi.org/10.21273/ HORTTECH.21.5.569.
Moreno, I. E.; Ojeda, B. C.; Avila, Q. G.; Prieto, G. B.; Parra, Q. R. and Anchondo, R. T. 2015. Sodium sulfate exposure slows growth of native pecan seedlings. Rev. Inter. Bot. Exp. 84(1):80-85. https://Doi:10.32604/phyton.2015.84.080.
Muhsin, T. M. and Zwiazek, J. J. 2002. Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodium uptake in white spruce (Picea glauca) seedlings. Plant and Soil 238(2):217-225. Doi: https://doi.org/10.1023/A:1014435 407735.
Nguyen, M.; Calvo, P. M. and Zwiazek, J. 2006. Gas exchange and growth responses of ectomycorrhizal Picea mariana, Picea glauca, and Pinus banksiana seedlings to NaCl and Na2SO4. Plant Biol. 8(5):646-652. Doi: https://doi.org/10.1055/s-2006-924106.
Parvin, S.; Van, G. M.; Yeasmin, T.; Verbruggen, E. and Honnay, O. 2020. Effects of single and multiple species inocula of arbuscular mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza. Doi: https://doi.org/10.1007/ s00572-020-00957-9. 30(4):431-444.
Porcel, R.; Aroca, R.; Azcon, R. and Ruiz, L. J. M. 2016. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza. 26(7):673-684. Doi: https://doi.org/10.1007/s00572-016-0704-5.
Ruiz. L. J. M.; Porcel R.; Azcon, C. and Aroca, R. 2012. Regulation of arbuscular mycorrhizae of the integrated physiological response to salinity in plants: new challenges in physiological and molecular studies. J. Exp. Bot. 63(11):4033-4044. Doi: https://doi.org/10.1093/jxb/ ers126.
Scagel, C. F.; Bryla, D. R. and Lee, J. 2017. Salt exclusion and mycorrhizal symbiosis increase tolerance to NaCl and KCl2 salinity in ‘Siam Queen’ basil. HortScience. 52(2):278-287. Doi: https://doi.org/10.21273/hortsci11256-16.
Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F. and Huang, Y. 2008. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza. 18(6):287-296. Doi: https://doi.org/10.1007/s00572-008-0180-7.
Smith, S. E.; Facelli, E.; Pope, S. and Smith, F. A. 2010. Plant performance in stressful environments: interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant and Soil. 326(1):3-20. Doi: https://doi.org/10.1007/s11104-009-9981-5.
Syvertsen, J. and Levy, Y. 2005. Salinity interactions with other abiotic and biotic stresses in citrus. HortTechnology. 15(1):100-103. Doi: https://doi.org/10.21273/horttech.15.1. 0100.
Yang, S. J.; Zhang, Z. L.; Xue, Y. X.; Zhang, Z. F. and Shi, S. Y. 2014. Arbuscular mycorrhizal fungi increase salt tolerance of Apple seedlings. Bot. Stud. 55(70):1-7. Doi: https://doi.org/10.1186/s40529-014-0070-6.
Yi, H.; Calvo, P. M.; MacKinnon, M. D. and Zwiazek, J. J. 2008. Responses of ectomycorrhizal Populus tremuloides and Betula papyrifera seedlings to salinity. Environ. Exp. Bot. 62(3):357-363. Doi: https://doi.org/10.1016/j.envexpbot.2007.10.008
Zwiazek, J. J.; Equiza, M. A.; Karst, J.; Senorans, J.; Wartenbe, M. and Calvo, P. M. 2019. Role of urban ectomycorrhizal fungi in improving the tolerance of lodgepole pine (Pinus contorta) seedlings to salt stress. Mycorrhiza. 29(4):303-312. Doi: https://doi.org/10.1007/ s00572-019-00893-3.
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


