PVA-Chitosan-nCu complex improves yield and defense response in tomato
DOI:
https://doi.org/10.29312/remexca.v12i6.3012Keywords:
biostimulant, gene expression, stress, vegetablesAbstract
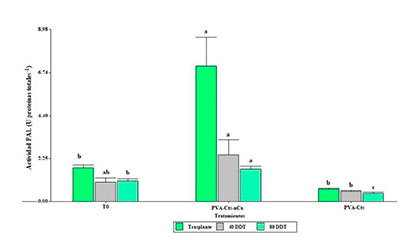
Currently the use of nanotechnology is revolutionizing agricultural production. Copper nanoparticles have been shown to influence the growth and development of different plant species, in addition to operating as stress resistance inducers. The objective of the present research was to evaluate the response in growth and yield, as well as the activation of the defense system of tomato plants. The treatments evaluated were a complex of polyvinyl alcohol-chitosan-copper nanoparticles (PVA-Cts-nCu), another complex of PVA-Cts and an absolute control (T0). The treatments were applied via foliar in tomato plants under greenhouse conditions. During the crop cycle, agronomic variables were determined, and the activity of enzymes related to stress tolerance such as β-1,3 glucanase, chitinase and phenylalanine ammonia lyase (PAL), as well as the expression of the PR1 gene. The PVA-Cts-nCu complex increased yield, number of fruits, average fruit weight, aerial fresh weight and root fresh weight, in addition, it promoted the defense system by increasing the PAL enzyme activity, as well as the overexpression of the PR1 gene.
Downloads
References
AbuQamar, S.; Luo, H.; Laluk, K.; Mickelbart, M. V. and Mengiste, T. 2009. Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor. The Plant J. 58(2):347-360. DOI: https://doi.org/10.1111/j.1365-313X.2008.03783.x
Adhikari, T.; Kundu, S.; Biswas, A. K.; Tarafdar, J. C. and Rao, A. S. 2012. Effect of copper oxide nano particle on seed germination of selected crops. J. Agric. Sci. Technol. 2(6A):815-823.
Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K. and Acharya, K. 2015. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Scientific reports. 5(1):1-14. DOI: https://doi.org/10.1038/srep15195
Chun, S. C. and Chandrasekaran, M. 2019. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Inter. J. Biol. Macromol. 125(51):948-954.
Cumplido-Nájera, C. F.; González-Morales, S.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2019. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. 245(1):82-89.
El Hadrami, A.; Adam, L. R.; El Hadrami, I. and Daayf, F. 2010. Chitosan in plant protection. Marine drugs. 8(4):968-987. DOI: https://doi.org/10.3390/md8040968
Falcón-Rodríguez, A.; Costales-Menéndez, D.; Martínez-Téllez, M. Á. y Gordon, T. A. 2012. Respuesta enzimática y de crecimiento en una variedad comercial de tabaco (Nicotiana tabacum, L.) tratada por aspersión foliar de un polímero de quitosana. Cultivos Tropicales. 33(1):65-70.
Fatima, F.; Hashim, A. and Anees, S. 2021. Efficacy of nanoparticles as nanofertilizer production: a review. Environ. Sci. Pollut. Res. 28(2):1292-1303.
Fish, W. W.; Perkins-Veazie, P. and Collins, J. K. 2002. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Composition and Analysis. 15(3):309-317. DOI: https://doi.org/10.1006/jfca.2002.1069
González-Peña, D.; Costales, D. y Falcón, A. B. 2014. Influencia de un polímero de quitosana en el crecimiento y la actividad de enzimas defensivas en tomate (Solanum lycopersicum L.). Cultivos Tropicales. 35(1):35-42.
Hernández, H H.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Hernández-Fuentes, A. D. and Juárez-Maldonado, A. 2017. Cu Nanoparticles in chitosan-PVA hydrogels as promoters of growth, productivity and fruit quality in tomato. Emirates J. Food Agric. 29(8):573-580.
Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G. and Juárez-Maldonado, A. 2018a. Effects of chitosan-PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules. 23(1):178-192. DOI: https://doi.org/10.3390/molecules23010178
Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D. and González-Morales, S. 2018b. Chitosan-PVA and copper nanoparticles improve growth and overexpress the SOD and JA genes in tomato plants under salt stress. Agronomy. 8(9):175-185.
Kasana, R. C.; Panwar, N. R.; Kaul, R. K. and Kumar, P. 2017. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Letters. 15(2):233-240. DOI: https://doi.org/10.1007/s10311-017-0615-5
López-Vargas, E. R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2018. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 8(7):1020-1034.
Padayatt, S. J.; Daruwala, R.; Wang, Y.; Eck, P. K.; Song, J.; Koh, W. S. and Levine, M. 2001. Vitamin C: from molecular actions to optimum intake. Handbook of antioxidants. CRC Press. Washington, DC. USA. 117-145. DOI: https://doi.org/10.1201/9780203904046.pt3
Pérez-Labrada, F.; López-Vargas, E. R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A. and Juárez-Maldonado, A. 2019. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants. 8(6):151-167.
Pestovsky, Y. S. and Martínez-Antonio, A. 2017. The use of nanoparticles and nanoformulations in agriculture. J. Nanoscie. Nanotechnol. 17(12):8699-8730. DOI: https://doi.org/10.1166/jnn.2017.15041
Pinedo-Guerrero, Z. H.; Hernández-Fuentes, A. D.; Ortega-Ortiz, H.; Benavides-Mendoza, A. and Cadenas-Pliego, G. 2017. Cu nanoparticles in hydrogels of chitosan-PVA affects the characteristics of post-harvest and bioactive compounds of jalapeño pepper. Molecules. 22(6):926. DOI: https://doi.org/10.3390/molecules22060926
Pradhan, S.; Patra, P.; Mitra, S.; Dey, K. K.; Basu, S.; Chandra, S.; and Goswami, A. 2015. Copper nanoparticle (CuNP) nanochain arrays with a reduced toxicity response: a biophysical and biochemical outlook on vigna radiata. J. Agric. Food Chem. 63(10):2606-2617. DOI: https://doi.org/10.1021/jf504614w
Rajput, V. D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V. and Fedorenko, A. 2018. Effects of copper nanoparticles (CuO NPs) on crop plants: a mini review. Bionanoscience. 8(1):36-42. DOI: https://doi.org/10.1007/s12668-017-0466-3
Ramos, S. J.; Faquin, V.; Guilherme, L. R. G.; Castro, E. M.; Ávila, F. W.; Carvalho, G. S. and Oliveira, C. 2010. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 56(12):584-588. DOI: https://doi.org/10.17221/113/2010-PSE
Rodríguez, A. T.; Ramírez, M. A.; Falcón, A.; Utria, E. y Bautista, S. 2006. Estimulacion de algunas enzimas en plantas de arroz (Oryza sativa, L.) tratadas con un hidrolizado de quitosana. Cultivos Tropicales. 27(2):87-91.
Rodríguez-Pedroso, A. T.; Ramírez-Arrebato, M. Á.; Cárdenas-Travieso, R. M.; Falcón-Rodríguez, A. y Bautista-Baños, S. 2006. Efecto de la quitosana en la inducción de la actividad de enzimas relacionadas con la defensa y protección de plántulas de arroz (Oryza sativa L.) contra Pyricularia grisea Sacc. Rev. Mex. Fitopatol. 24(1):1-7.
Rodríguez-Pedroso, A. T.; Ramírez-Arrebato, M. A.; Rivero-González, D.; Bosquez-Molina, E.; Barrera-Necha, L. L. y Bautista-Baños, S. 2009. Propiedades químico-estructurales y actividad biológica de la quitosana en microorganismos fitopatógenos. Rev. Chapingo. Ser. Hortic. 15(3):307-317.
Rouphael, Y. and Colla, G. 2020. Biostimulants in agriculture. Frontiers in Plant Sci. 11(1):40-47.
Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M. K.; Sharma, S. S.; Pal, A. and Biswas, P. 2015. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Inter. J. Biol. Macromol. 75(1):346-353. DOI: https://doi.org/10.1016/j.ijbiomac.2015.01.027
Sánchez, G. R.; Mercado, E. C.; Peña, E. B.; Cruz, H. R. y Pineda, E. G. 2010. El ácido salicílico y su participación en la resistencia a patógenos en plantas. Biológicas. 12(2):90-95.
Santos-Sánchez, N. F.; Salas-Coronado, R.; Villanueva-Cañongo, C. and Hernández-Carlos, B. 2019. Antioxidant compounds and their antioxidant mechanism. London, UK. IntechOpen. 1-28 p.
Sierra-Ávila, R. M. P.; Cadenas-Pliego, G.; Avila-Orta, C.; Betancourt, R.; Jiménez-Regalado, E.; Jiménez-Barrera, R. and Martínez, G. 2014. Synthesis of copper nanoparticles coated with nitrogen ligands. J. Nanomater. 2014(1):74-82. DOI: https://doi.org/10.1155/2014/361791
Sierra-Ávila, R.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Comparán Padilla, V.; Ávila-Orta, C.; Pérez Camacho, O.; Jiménez-Regalado, E.; Hernández-Hernández, E. and Jiménez-Barrera, R. M. 2015. Synthesis of copper nanoparticles using mixture of allylamine and polyallylamine. J. Nanomaterials. 361797(1):1-8. DOI: https://doi.org/10.1155/2015/367341
Somasundaran, P.; Fang, X.; Ponnurangam, S. and Li, B. 2010. Nanoparticles: Characteristics, mechanisms and modulation of biotoxicity. KONA powder and particle journal. 28(1):38-49. DOI: https://doi.org/10.14356/kona.2010007
Steiner, A. A. 1961. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil. 15(2):134-154. DOI: https://doi.org/10.1007/BF01347224
Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M. R.; Kawiak, A.; Jeziorek, M.; Wyderska, S. and Chinou, I. 2012. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. In vitro Cellular & Developmental Biology-Plant. 48(5):555-564. DOI: https://doi.org/10.1007/s11627-012-9443-2
Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S. A.; ur Rehman, H. and Sanaullah, M. 2020. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 721(1):137778.
Yang, W.; Fortunati, E.; Bertoglio, F.; Owczarek, J. S.; Bruni, G.; Kozanecki, M. and Puglia, D. 2018. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydrate Polymers. 181(1):275-284. DOI: https://doi.org/10.1016/j.carbpol.2017.10.084
Yang, W.; Owczarek, J. S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G. M. adn Puglia, D. 2016. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Industrial Crops and Products. 94(1):800-811. DOI: https://doi.org/10.1016/j.indcrop.2016.09.061
Yu, Z. and Dahlgren, R. A. 2000. Evaluation of methods for measuring polyphenols in conifer foliage. J. Chem. Ecol. 26(9):2119-2140. DOI: https://doi.org/10.1023/A:1005568416040
Zhang, X. and Liu, C. J. 2015. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant. 8(1):17-27. DOI: https://doi.org/10.1016/j.molp.2014.11.001
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


