Use of Bacillus sp. as a resistance inducer in prickly pear against ‘scaly rot’
DOI:
https://doi.org/10.29312/remexca.v14i1.2898Keywords:
Bacillus amyloliquefaciens, Neoscytalidium hyalinum, Opuntia ficus-indica (L.) Mill., antagonism, resistance induction, scaly rotAbstract
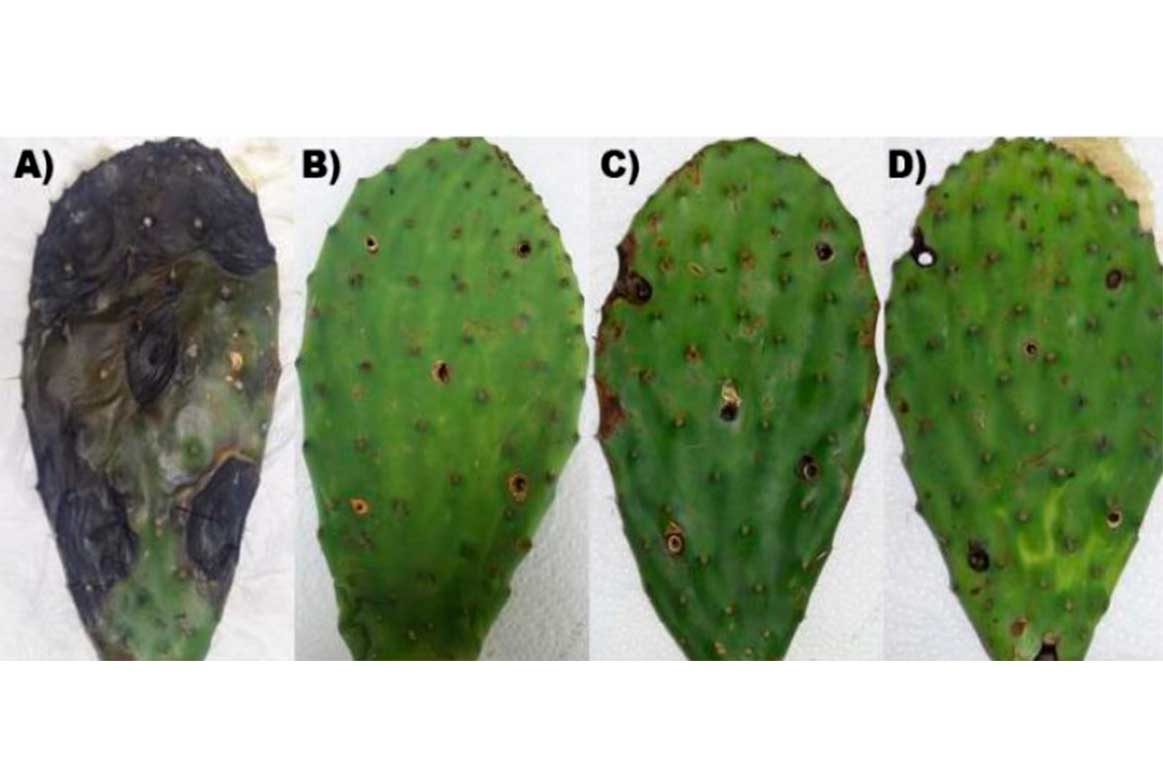
‘Scaly rot’ is one of the most important diseases in prickly pear, for this reason the objective of the present work was to isolate and identify fungi associated with ‘scaly rot’ and evaluate the ability of bacteria of the genus Bacillus sp. isolated to avoid the disease of ‘scaly rot’ in prickly pear [Opuntia ficus-indica (L.) Mill.]. Ten bacteria of the genus Bacillus sp. were analyzed in in vitro antagonism tests against the pathogen Neoscytalidium hyalinum, which was identified as the causative agent of ‘scaly rot’. The results of this study demonstrated that all isolates of Bacillus sp. significantly decreased (p≤ 0.05) the radius of the pathogen in the in vitro test, with isolates G11, G21 and G31 standing out. In the in vivo resistance induction tests, isolates G11, G21 and G31 significantly decreased (p≤ 0.05) the diameter of the lesions caused by Neoscytalidium hyalinum. The identification of the species used was carried out through a phylogenetic analysis of the sequences obtained of the regions 16s rRNA (bacteria) and ITS1-5.8s-ITS2 (fungi). Bacterial isolates G11, G21 and G31 were identified as Bacillus amyloliquefaciens and all fungi belonging to the genus Neoscytalidium sp. were identified as N. hyalinum.
Downloads
References
Ammar, M. I.; Shltout, A. M. and Kamhawy, M. A. 2004. Cladode and fruit rots of prickly pear (Opuntia ficus-indica L. Mill.) in Egypt. Egypt. J. Phytopathol. 32(1-2):119-128.
Canchignia, M. H. F.; Pinargote, C. B.; Peñafiel, J. M.; Carranza, P. M. S.; Prieto, B. O. y Gaibor, F. R. 2015. Respuesta de poblaciones microbianas que lideran el crecimiento en raíces y resistencia sistémica inducida. Ciencia y Tecnología. 8(2):1-11.
Chowdappa, P.; Mohan, K. S. P.; Jyothi, L. M. and Upreti, K. K. 2013. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biological Control. 65(1):109-117.
Chowdhury, S. P.; Hartmann, A.; Gao, X. W. and Borriss, R. 2015. Biocontrol mechanism by root associated Bacillus amyloliquefaciens FZB42 - A review. Front. Microbiol. 6(780):1-11.
De Vos, P.; Garrity, G. M.; Jones, D.; Krieg, N. R.; Ludwig, W.; Rainy, F. A. and Whitman, W. B. 2009. Bergey’s manual of systematic bacteriology-second. 3ra. Ed. The Firmicutes. Springer-Verlag New York. New York. USA. 1445 p.
Deising, H. B.; Reimann, S. and Pascholati, S. F. 2008. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 39(2):286-295.
Días, P. L. N. 2012. Systemic acquired resistance induced by salicylic acid. Biotecnología en el sector agropecuario y agroindustrial. 10(2):257-267.
Durrant, W. E. and Dong, X. 2004. Systemic acquired resistance. Ann. Rev. Phytopathol. 42(1):185-209.
Faedda, R.; D’Aquino, S.; Granata, G.; Pane, A.; Palma, A., Sanzani, S. M. and Cacciola, S. O. 2016. Postharvest fungal diseases of cactus pear fruit in southern Italy. Acta Hortic. 1144:215-218.
Feijo, F. M.; Silva, M. J. S.; Nascimento, A. D.; Infante, N. B.; Ramos, S. R.; Assunção, I. P. and Lima, G. S. A. 2019. Botryosphaeriaceae species associated with the pickly pear cactus, Nopalea cochenillifera. Trop. Plant Pathol. 44:452-459.
Flores, F. R.; Velázquez, V. M. G.; León, R. R.; Flores, M. H. E. and Hernández, L. A. N. 2013. Identification of fungal species associated with cladode spot of prickly pear and their sensitivity to chitosan. J. Phytopathol. 161(7-8):544-552.
Forouhar, F.; Yang, Y.; Kumar, D.; Chen, Y.; Fridman, E.; Park, S. W. and Tong, L. 2005. Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proceedings of the National Academy of Sciences of the United States of America. 102(5):1773-1778.
Gond, S. K.; Bergen, M. S.; Torres, M. S. and White, J. F. 2015. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 172:79-87.
Guo, J. R.; Schnieder, F.; Abd-Elsalam, K. A. and Verreet, J. A. 2005. Rapid and efficient extraction of genomic DNA from different phytopathogenic fungi using DNAzol reagent. Biotechnology Letters. 27(1):3-6.
Layton, C.; Maldonado, E.; Monroy, L.; Corrales, L. C. and Sánchez, L. C. 2017. Bacillus spp.; perspectiva de su efecto biocontrolador mediante antibiosis en cultivos afectados por fitopatógenos. Nova. 9(16):177.
Lu, J. J.; Perng, C. L.; Lee, S. Y. and Wan, C. C. 2000. Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J. Clinical Microbiol. 38(6):2076-2080.
Machado, A. R.; Pinho, D. B.; De Oliveira, S. A. S. and Pereira, O. L. 2014. New occurrences of Botryosphaeriaceae causing black root rot of cassava in Brazil. Trop. Plant Pathol. 39(6):464-470.
Méndez, G. S. J.; Magaña, D. T. y Herrera, J. G. E. 2007. Identificación y control de las enfermedades más comunes en el nopal. Rev. Salud Pública y Nutrición. 14:105-113.
Mohammadi, P.; Tozlu, E.; Kotan, R. and Şenol, K. M. 2017. Potential of some bacteria for biological control of postharvest citrus green mould caused by Penicillium digitatum. Plant Protec. Sci. 53(3):134-143.
Moo-Koh, F. A.; Cristóbal-Alejo, J.; Reyes-Ramírez, A.; Tun-Suarez, J. M. y Gamboa-Angulo, M. 2017. Identificación molecular de aislados de Trichoderma spp. y su actividad promotora en Solanum lycopersicum L. Investigación y ciencia de la Universidad Autónoma de Aguascalientes. 25(72):5-11.
Nakkeeran, S.; Kavitha, K.; Chandrasekar, G.; Renukadevi, P. and Fernando, W. G. D. 2007. Induction of plant defence compounds by Pseudomonas chlororaphis PA23 and Bacillus subtilis BSCBE4 in controlling damping-off of hot pepper caused by Pythium aphanidermatum. Bio. Sci. Technol. 16(4):403-416.
Ongena, M.; Duby, F.; Jourdan, E.; Beaudry, T.; Jadin, V.; Dommes, J. and Thonart, P. 2005. Bacillus subtilis M4 decreases plant susceptibility towards fungal pathogens by increasing host resistance associated with differential gene expression. Appl. Microbiol. Biotechnol. 67(5):692-698.
Ordeñana, K. M. 1998. Mecanismos de defensa en las interacciones planta-patógeno. Manejo Integrado de Plagas. 63:22-32.
Phillips, A. J. L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M. J.; Groenewald, J. Z. and Crous, P. W. 2013. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 76:51-167.
Reyes-Ramirez, A.; Ruiz-Sánchez, E.; Yam-Chimal, C. y Dzul-Chan, M. 2011. Selección de Bacillus spp. con actividad antagónica in vitro contra Macrophomina phaseolina (Tassi) Goid. en diferentes medios de cultivo. Fitosanidad. 15(2):117-121.
Ruiz, S. E.; Mejía, B. M. A.; Cristóbal, A. J.; Valencia, B. A. y Reyes, R. A. 2014. Actividad antagónica de filtrados de Bacillus subtilis contra Colletotrichum gloeosporioides (Penz.) Antagonistic activity of Bacillus subtilis vs Colletotrichum gloeosporioides (Penz.) resumen introducción. Rev. Mex. Cienc. Agríc. 5(7):1325-1332.
Santoyo, G.; Orozco, M. M. del C. and Govindappa, M. 2012. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Bio. Sci. Technol. 22(8):855-872.
Souza, A. E. F.; Nascimento, L. C. and Souza, B. O. DE. 2017. Principal Components of the intensity of squamous rot on prickly pear plantations in the semiarid region of the State of Paraíba, Brazil. Rev. Caatinga. 30(2):370-376.
Souza, A. E. F.; Nascimento, L. C.; Araujo, E.; López, E. B. and Souto, F. M. 2010. Ocorrencia e identificacao dos agentes etiologicos de doencas em palma forrageira (Opuntia ficus-indica Mill.) no semiárido paraibo. Biotemas. 23(3):11-20.
Spadaro, D. and Gullino, M. L. 2004. State of the art and future prospects of the biological control of postharvest fruit diseases. Inter. J. Food Microbiol. 91(2):185-194.
Swart, W. J. and Kriel, W. M. 2002. Pathogens associated with necrosis of cactus pear cladodes in south Africa. Plant Dis. 86(6):693-693.
Swart, W. J.; Oelofse, R. M. and Labuschagne, M. T. 2003. Susceptibility of south African cactus pear varieties to four fungi commonly associated with disease symptoms. J. Professional Association for Cactus Development. 5:86-97.
Valiente, M. y Pavone, D. 2013. Identificación de cepas del hongo Trichoderma spp. Por métodos moleculares. Faraute. 8(2):1-9.
Vallad, G. E. and Goodman, R. M. 2004. Systemic acquired resistance and induced systemic resistance in conventional Agriculture. Crop Sci. Soc. Am. 44:1920-1934.
Villarreal, D. M. F.; Villa, R. E. D.; Cira, Ch. L. A.; Estrada, A. M. I.; Parra, C. F. I. y De los Santos, V. S. 2018. El género Bacillus como agente de control biológico y sus implicaciones en la bioseguridad agrícola. Rev. Mex. Fitopatol. 36(1):95-130.
Wang, X.; Wang, L.; Wang, J.; Jin, P.; Liu, H. and Zheng, Y. 2014. Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE. 9(11):3-10.
Watanabe, T. 2010. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. Third Edition. CRC Press Taylo and Francis Group. Boca Raton. Florida. USA. 426 p.
White, T. J.; Bruns, T. Lee, S. and Taylor, J. 1990. Amplification and direct secuencing of fungal ribosmal RNA genes for phylogenetics. In: Inns, M. A.; Gerlfand, D. H.; Sninsky J. J. and White, T. J. Ed. PCR Protocols: A Guide to methods and applications. San Diego, CA: Academic Press. San Diego. California. USA. 315-322 pp.
Wiesel, L.; Newton, A. C.; Elliott, I.; Booty, D.; Gilroy, E. M.; Birch, P. R. J. and Hein, I. 2014. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Frontiers in Plant Sci. 5:1-13.
Wilson, M. 1997. Biocontrol of aerial plant diseases in agriculture and horticulture: current approaches and future prospects. J. Ind. Microbiol. Biotechnol. 19(3):188-191.
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


