Phenolic compounds and antiradical capacity of five wild accessions of Portulaca oleracea L. obtained with three solvents
DOI:
https://doi.org/10.29312/remexca.v12i6.2729Keywords:
antioxidantes, planta comestible, variación fitoquímicosAbstract
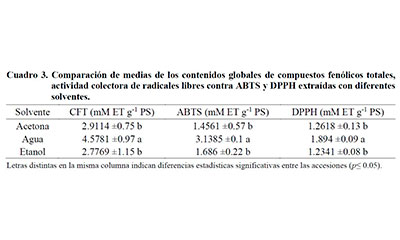
Purslane (Portulaca oleracea L.) is a species recognized for its high levels of bioactive compounds, among which the compounds with antioxidant properties stand out. The objective of the work was to determine the variation of phenolic metabolites and their antiradical capabilities in wild accessions of P. oleracea. The research was conducted with samples from five municipalities in Tamaulipas, Mexico collected in October 2018. The contents of total phenolic compounds (CFT) and free radical scavenging capacity against ABTS (2,2’-azino-bis- (3-ethylbenzothiazoline-6-sulfonic) acid) and DPPH (2,2-diphenyl-1-picrylhydracyl) were determined. Extractions were made from vegetative parts using three solvents: acetone, water and ethanol. The accession of the localities of Abasolo and Padilla were the ones that had the highest amount of CFT with 5.8938 ±0.03 and 5.3742 ±0.11 mEAG g-1 PS, respectively, using water in the extraction. Regarding the free radical scavenging capacity against ABTS, the accession of Abasolo and Jiménez recorded the highest values, with 3.27 ±0.06 and 3.2226 ±0.04 mM ET g-1 PS. Regarding the level against DPPH radicals, the accession of Abasolo was the highest with 2.0204 ±0.05 mM ET g-1 PS, using water in the extraction. Water was the best solvent for the extraction of the determined contents. Heterogeneity was observed in the composition and levels of the parameters evaluated among the accessions. Wild P. oleracea accessions represent reservoirs of phenolic compounds and free radical scavenging capacity, including cultivated and wild varieties.
Downloads
References
Alam, M.; Juraimi, A. S.; Rafii, M. Y.; Abdul, A.; Aslani, F.; Hasan, M. M.; Zainudin, M. A. and Uddin, M. 2014. Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. BioMed Res. Int. 2014(1):1-6. https://doi.org/10.1155/2014/951019. DOI: https://doi.org/10.1155/2014/296063
Allahmoradi, E.; Taghiloo, S.; Omrani-Nava, V.; Shobeiri, S. S.; Tehrani, M.; Ebrahimzadeh, M. A. and Asgarian-Omran, H. 2018. Antiinflammatory effects of the Portulaca oleracea hydroalcholic extract on human peripheral blood mononuclear cells. Med. J. Islam Repub. Iran. 32(1):1-6.
Almulaiky, Y. Q.; Aldhahri, M.; Al-abbasi, F. A.; Al-Harbi, S. A. and Shiboob, M. H. 2020. In Vitro Assessment of Antioxidant Enzymes, Phenolic Contents and Antioxidant Capacity of the Verdolaga (Portulacaceae). Int. J. Nutr. 4(4):36-47. https://doi.org/10.14302/ issn.2379-7835.ijn-19-3144.
Alu’datt, M. H.; Rababah, T.; Alhamad, M. N.; Al-Tawaha, A.; Al-Tawaha, A. R.; Gammoh, S.; Ereifeja, K. I.; Al-Karakic, G.; Hamashae, F.; Tranchantg, C. C. and Kubow, S. 2019. Herbal yield, nutritive composition, phenolic contents and antioxidant activity of purslane (Portulaca oleracea L.) grown in different soilless media in a closed system. Ind. Crop Prod. 141:11746. https://doi.org/10.1016/j.indcrop.2019.111746.
Araghi, A. M.; Nemati, H.; Azizi, M.; Moshtaghi, N.; Shoor, M. and Hadian, J. 2019. Assessment of phytochemical and agro-morphological variability among different wild accessions of Mentha longifolia L. cultivated in field condition. Ind. Crop. Prod. 140:111698. https://doi.org/10.1016/j.indcrop.2019.111698.
Assefa, A. D.; Keum, Y. S. and Saini, R. K. 2018. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 12(3):1548-1555. https://doi.org/10.1007/s11694-018-9770-z. DOI: https://doi.org/10.1007/s11694-018-9770-z
Bartolini, S.; Leccese, A.; Remorini, D.; Iacona, C. and Viti, R. 2018. Quality and antioxidant traits of organic apricots (Prunus armeniaca L.) at harvest and after storage. Eur. J. Hortic. Sci. 83(1):12-17. https://doi.org/10.17660/eJHS.2018/83.1.2. DOI: https://doi.org/10.17660/eJHS.2018/83.1.2
Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J. V.; Lull, C.; Donat, M. P.; Mayoral, O. and Vicente, O. 2016. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 38(1):1-15. https://doi.org/10.1007/s11738-015-2025-2. DOI: https://doi.org/10.1007/s11738-015-2025-2
Brand-Williams, W.; Cuvelier, M. E. and Berset, C. L. W. T. 1995. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 28(1):25-30. https://doi.org/ 10.1016/ S0023-6438(95)80008-5. DOI: https://doi.org/10.1016/S0023-6438(95)80008-5
Capecka, E.; Mareczek, A. and Leja, M. 2005. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 93(2):223-226. https://doi.org/10.1016/j.foodchem. 2004.09.020. DOI: https://doi.org/10.1016/j.foodchem.2004.09.020
Caverzan, A.; Casassola, A. and Brammer, S. P. 2016. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 39(1):1-6. https://doi.org/10.1590/1678-4685-GMB-2015-0109. DOI: https://doi.org/10.1590/1678-4685-GMB-2015-0109
Chan, C. L.; Gan, R. Y. and Corke, H. 2016. The phenolic composition and antioxidant capacity of soluble and bound extracts in selected dietary spices and medicinal herbs. Int. J. Food Sci. Tech. 51(3):565-573. https://doi.org/10.1111/ijfs.13024. DOI: https://doi.org/10.1111/ijfs.13024
Cobaleda-Velasco, M.; Alanis-Bañuelos, R. E.; Almaraz-Abarca, N.; Rojas-López, M.; González-Valdez, L. S.; Ávila-Reyes J. A. and Rodrigo S. 2017. Phenolic profiles and antioxidant properties of Physalis angulata L. as quality indicators. J. Pharm. Pharmacogn. Res. 5(2):114-128.
Felhi, S.; Daoud, A.; Hajlaoui, H.; Mnafgui, K.; Gharsallah, N. and Kadri, A. 2017. Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis of Ecballium elaterium seeds and peels fruits. Food Sci. Technol. 37(3):483-492. http://dx.doi.org/10.1590/1678-457x.23516. DOI: https://doi.org/10.1590/1678-457x.23516
Frontela, C.; Canali, R. y Virgili, F. 2010. Empleo de compuestos fenólicos en la dieta para modular la respuesta inflamatoria intestinal. Gastroenterol Hepatol. 33(4):307-312. https://doi.org/10.1016/j.gastrohep.2009.09.006. DOI: https://doi.org/10.1016/j.gastrohep.2009.09.006
Gallo, M.; Conte, E. and Naviglio, D. 2017. Analysis and comparison of the antioxidant component of Portulaca oleracea leaves obtained by different solid-liquid extraction techniques. Antioxidants. 6(3):64. https://doi.org/10.3390/antiox6030064. DOI: https://doi.org/10.3390/antiox6030064
García, E. 2004. Modificaciones al sistema de clasificación climática de Köppen. 5a. (Ed.). Instituto de Geografía-Universidad Nacional Autónoma de México (UNAM). DF, México. 92 p.
Gatea, F.; Teodor, E. D.; Seciu, A. M.; Nagodă, E. and Radu, G. L. 2017. Chemical constituents and bioactive potential of Portulaca pilosa L vs. Portulaca oleracea L. Med. Chem. Res. 26(7):1516-1527. https://doi.org/10.1007/s00044-017-1862-5. DOI: https://doi.org/10.1007/s00044-017-1862-5
Gharibi, S.; Tabatabaei, B. E. S.; Saeidi, G. and Goli, S. A. H. 2016. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 178(4):796-809. https://doi.org/10.1007/s12010-015-1909-3. DOI: https://doi.org/10.1007/s12010-015-1909-3
Gutiérrez, Á.; Ledesma, L.; García, I. y Grajales, O. 2007. Capacidad antioxidante total en alimentos convencionales y regionales de Chiapas, México. Rev. Cub. Salud Pública. 33(1):1-7. DOI: https://doi.org/10.1590/S0864-34662007000100008
Habibian, M.; Sadeghi, G. and Karimi, A. 2020. Phytochemicals and Antioxidant Properties of Solvent Extracts from Purslane (Portulaca oleracea L.): a preliminary study. Food Sci. Eng. 1(1):1-12. https://doi.org/10.37256/fse.11202046.
Hammer, O.; Harper, D. A. T. and Ryan, P. D. 2001. PAST: paleontological statistics software package for education and data analysis. Paleontol. Electron. 4(1):1-9.
Kaur, D.; Grewal, S. K.; Kaur, J. and Singh, S. 2017. Free radical scavenging activities can mitigate the effect of water stress in chickpea. Crop Pasture Sci. 68(6):544-554. https://doi.org/10.1071/CP17022. DOI: https://doi.org/10.1071/CP17022
Khodadadi, H.; Pakdel, R.; Khazaei, M.; Niazmand, S.; Bavarsad, K. and Hadjzadeh, M. 2018. A comparison of the effects of Portulaca oleracea seeds hydro-alcoholic extract and Vitamin C on biochemical, hemodynamic and functional parameters in cardiac tissue of rats with subclinical hyperthyroidism. Avicenna J. Phytomed. 8(2):161-169.
Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D. C.; Crişan, G. and Rohn, S. 2017. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: phytochemical characterization, biological profile, and computational studies. J. Enzyme Inhib. Med. Chem. 32(1):153-168. https://doi.org/10.1080/14756366.2016.1243535. DOI: https://doi.org/10.1080/14756366.2016.1243535
Nemzer, B.; Al-Taher, F. and Abshiru, N. 2020. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (Portulaca oleracea L.) genotypes. Food Chem. 320(1):126621. https://doi.org/10.1016/j.foodchem.2020. 126621.
Ninfali, P.; Mea, G.; Giorgini, S.; Rocchi, M. and Bacchiocca, M. 2005. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 93(2):257-266. https://doi.org/10.1079/BJN20041327. DOI: https://doi.org/10.1079/BJN20041327
Oliveira, I.; Valentao, P.; Lopes, R.; Andrade, P. B.; Bento, A. and Pereira, J. A. 2009. Phytochemical characterization and radical scanvenging activity of Portulaca oleracea L. leaves and Stems. Microchem. 92(2):129-134. https://doi.org/10.1016/j.microc.2009. 02.006. DOI: https://doi.org/10.1016/j.microc.2009.02.006
Qasim, M.; Abideen, Z.; Adnan, M. Y.; Gulzar, S.; Gul, B.; Rasheed, M. and Khan, M. A. 2017. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. African J. Bot. 110(1):240-250. https://doi.org/10.1016/j.sajb.2016.10.005. DOI: https://doi.org/10.1016/j.sajb.2016.10.005
Ramadan, B. K.; Schaalan, M. F. and Tolba, A. M. 2017. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement. Altern. Med. 17(1):37. https://doi.org/10.1186/s12906-016-1530-1. DOI: https://doi.org/10.1186/s12906-016-1530-1
Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M. and Rice-Evans, C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26(9-10):1231-1237. https://doi.org/10.1016/S0891-5849(98)00315-3. DOI: https://doi.org/10.1016/S0891-5849(98)00315-3
Román, N. R.; Del Rosario, M.; Castillo, A. M.; Sahagún, J. y Jiménez, M. A. 2018. Características nutricionales y nutracéuticas de hortalizas de uso ancestral en México. Rev. Fitotec. Mex. 41(3):245-253. https://doi.org/10.35196/rfm.2018.3.245-253.
Santiago-Saenz, Y. O.; Hernández-Fuentes, A. D.; Monroy-Torres, R.; Cariño-Cortés, R. and Jiménez-Alvarado, R. 2018. Physicochemical, nutritional and antioxidant characterization of three vegetables (Amaranthus hybridus L., Chenopodium berlandieri L., Portulaca oleracea L.) as potential sources of phytochemicals and bioactive compounds. J. Food Meas. Charact. 12(4):2855-2864. https://doi.org/10.1007/s11694-018-9900-7.
Sauceda, A. E. Q.; Palafox, H.; Sánchez, R. M. R. y Aguilar, G. A. G. 2011. Interacción de compuestos fenólicos y fibra dietaria: capacidad antioxidante y biodisponibilidad. Biotecnia. 13(3):3-11. https://doi.org/10.18633/bt.v13i3.91. DOI: https://doi.org/10.18633/bt.v13i3.91
Sdouga, D.; Branca, F.; Kabtni, S.; Trifi-Farah, N. and Marghali, S. 2020. Polyphenol variability of Italian and Tunisian populations of Portulaca oleracea L. Acta Hortic. 1267(1):115-118. https://doi.org/10.17660/ActaHortic.2020.1267.18.
Sicari, V.; Loizzo, M. R.; Tundis, R.; Mincione, A. and Pellicano, T. M. 2018. Portulaca oleracea L. (Purslane) extracts display antioxidant and hypoglycaemic effects. J. Appl. Bot. Food Qual. 91(1):39-46. https://doi.org/10.5073/JABFQ.2018.091.006.
Singleton, V. L.; Orthofer, R. and Lamuela-Raventós, R. M. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 299(1):152-178. Academic press. https://doi.org/10.1016/S0076-6879(99)99017-1. DOI: https://doi.org/10.1016/S0076-6879(99)99017-1
Thouri, A.; Chahdoura, H.; El Arem, A.; Hichri, A. O.; Hassin, R. B. and Achour, L. 2017. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti). BMC Complement. Altern. Med. 17(1):248. https://doi.org/10.1186/s12906-017-1751-y. DOI: https://doi.org/10.1186/s12906-017-1751-y
Top, O.; Bar, C.; Ökmen, B.; Özer, D. Y.; Rusçuklu, D.; Tamer, N.; Frary, A. and Doğanlar, S. 2014. Exploration of three solanum species for improvement of antioxidant traits in tomato. HortScience. 49(8):1003-1009. https://doi.org/10.21273/HORTSCI.49.8.1003.
Urbizu-González, A. L.; Castillo-Ruiz, O.; Martínez-Ávila, G. C. G. and Torres-Castillo, J. A. 2017. Natural variability of essential oil and antioxidants in the medicinal plant Turnera diffusa. Asian Pac. J. Trop. Med. 10(2):121-125. https://doi.org/10.1016/j.apjtm.2017. 01.013. DOI: https://doi.org/10.1016/j.apjtm.2017.01.013
Van Treuren, R.; Van Eekelen, H. D.; Wehrens, R. and de Vos, R. C. 2018. Metabolite variation in the lettuce gene pool: towards healthier crop varieties and food. Metabolomics. 14(11):146. https://doi.org/10.1007/s11306-018-1443-8.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Revista Mexicana de Ciencias Agrícolas

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Revista Mexicana de Ciencias Agrícolas accept the following conditions:
In accordance with copyright laws, Revista Mexicana de Ciencias Agrícolas recognizes and respects the authors’ moral right and ownership of property rights which will be transferred to the journal for dissemination in open access. Invariably, all the authors have to sign a letter of transfer of property rights and of originality of the article to Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) [National Institute of Forestry, Agricultural and Livestock Research]. The author(s) must pay a fee for the reception of articles before proceeding to editorial review.
All the texts published by Revista Mexicana de Ciencias Agrícolas —with no exception— are distributed under a Creative Commons License Attribution-NonCommercial 4.0 International (CC BY-NC 4.0), which allows third parties to use the publication as long as the work’s authorship and its first publication in this journal are mentioned.
The author(s) can enter into independent and additional contractual agreements for the nonexclusive distribution of the version of the article published in Revista Mexicana de Ciencias Agrícolas (for example include it into an institutional repository or publish it in a book) as long as it is clearly and explicitly indicated that the work was published for the first time in Revista Mexicana de Ciencias Agrícolas.
For all the above, the authors shall send the Letter-transfer of Property Rights for the first publication duly filled in and signed by the author(s). This form must be sent as a PDF file to: revista_atm@yahoo.com.mx; cienciasagricola@inifap.gob.mx; remexca2017@gmail.
This work is licensed under a Creative Commons Attribution-Noncommercial 4.0 International license.


